Christophe Boesch, Dasa Bombjakova, Adam Boyette & Amelia Meier
Technical intelligence and culture: Nut cracking in humans and chimpanzees
1.1 Background information about nut cracking
1.1.2.1 The Aka of the Central African Republic
1.1.2.2 The Mbendjele from the Republic of Congo
1.1.2.3 The Taï chimpanzees from Cote d^ ’Ivoire
2.1 Data collection procedures
2.2 Data analysis and statistical analysis
3.1 Material used to crack the nuts
3.1.1 Same nuts—Different tools
3.1.2 Different nuts—Same tools
3.2.1 Same nuts—Different tools
3.2.2 Different nuts—Same tools
3.3 Efficiency of nut-cracking technique
3.3.1 Same nut—different tools
3.3.2 Different nuts—Same tools
4.1 Contrasting chimpanzee and human nut-cracking technique
4.2 Technical intelligence and nut cracking
Christophe Boesch[1]{1} | Dasa Bombjakova[2]{2} | Adam Boyette[3] | Amelia Meier[4]
Correspondence
Christophe Boesch, Department of Primatology, Max Planck Institute of Evolutionary Anthropology, Deutscher Platz 6, 04103 Leipzig, Germany.
Email: boesch@eva.mpg.de
Funding information
Max Planck Society
Abstract
Objectives: According to the technical intelligence hypothesis, humans are superior to all other animal species in understanding and using tools. However, the vast majority of comparative studies between humans and chimpanzees, both proficient tool users, have not controlled for the effects of age, prior knowledge, past experience, rearing conditions, or differences in experimental procedures. We tested whether humans are superior to chimpanzees in selecting better tools, using them more dexteriously, achieving higher performance and gaining access to more resource as predicted under the technical intelligence hypothesis.
Materials and methods: Aka and Mbendjele hunter-gatherers in the rainforest of Central African Republic and the Republic of Congo, respectively, and Taï chimpanzees in the rainforest of Cote^ d’Ivoire were observed cracking hard Panda oleosa nuts with different tools, as well as the soft Coula edulis and Elaeis guinensis nuts. The nut-cracking techniques, hammer material selection and two efficiency measures were compared.
Results: As predicted, the Aka and the Mbendjele were able to exploit more species of hard nuts in the forest than chimpanzees. However, the chimpanzees were sometimes more efficient than the humans. Social roles differed between the two species, with the Aka and especially the Mbendjele exhibiting cooperation between nut-crackers whereas the chimpanzees were mainly individualistic.
Discussion: Observations of nut-cracking by humans and chimpanzees only partially supported the technical intelligence hypothesis as higher degrees of flexibility in tool selection seen in chimpanzees compensated for use of less efficient tool material than in humans. Nut cracking was a stronger social undertaking in humans than in chimpanzees.
Keywords
chimpanzees, efficiency, humans, nut cracking, technical intelligence, tool use
1. Introduction
Humans have been proposed to possess a specific form of intelligence termed “technical intelligence” which has allowed the development of specialized skills in thinking, modifying and combining material objects, as well as using them to modify the outside world to serve their own interest (Johnson-Frey, 2003; Mithen, 1996; Oakley, 1956). These abilities, by freeing humans from environmental constraints, have allowed them to occupy most habitats and become the most successful animal species on Earth (e.g., Foley & Lahr, 2003; Leakey, 1980). In particular, a technical revolution concomitant with the emergence of Homo sapiens sapiens some 200,000 years ago found our ancestors developing a number of new abilities related to tools and artifacts (Leakey, 1980; Mellars & Stringer, 1989; but see McBrearty & Brooks, 2000 for a more gradual view of these changes). However, the question remains: Are these tool-specific skills an evolutionary innovation appearing uniquely in the hominid line, or are some of these skills shared in part or whole with the chimpanzee, a species known to be a prolific tool user throughout its distribution range (e.g., Boesch, 2012; Boesch & Boesch, 1990; Boesch & Boesch-Achermann, 2000; Goodall, 1970, 1986; Sanz & Morgan, 2007, 2009)?
This question has been discussed extensively but is difficult to address because it is challenging to use archeological remains to make inferences about technical intelligence (Ambrose, 2010; Bar-Yosef & van Peer, 2009; Beaune, 2004; Davidson & Noble, 1993; Dietrich, Toth, Schick, & Chaminade, 2008). For example, using the structures of core stones and flakes, it has been suggested that the Acheulean technology is clearly distinguished from the Oldowan technology by signs of symmetry in the production of tools (Wynn, 1993, 2002), while others reached different conclusions about the technical knowledge involved (see Davidson & Noble, 1993; Iovita & McPherron, 2011; McPherron, 2000). The stasis of the shape of the hand axe for almost one million years has been extensively discussed, but opinions are still divergent about its functions and means of production (e.g., Henshilwood, D’errico, Marean, Milo, & Yates, 2001; Iovita & McPherron, 2011; McPherron, 2000, 2013). Furthermore, the persistence of stone as compared to wood and bone artifacts may bias our understanding of the origins of tool use in early hominids (Lemorini et al., 2014).
In a comparative approach to address this question, the technical abilities of the chimpanzee have been studied both in the wild and in captivity with mixed results. Field observations revealed that all known wild populations of chimpanzees use different sets of tools with different shapes and materials to fulfill different purposes, including accessing important food sources (e.g., Boesch, 2012; Boesch & Boesch, 1990; Goodall, 1970, 1986; Sanz & Morgan, 2007, 2009; Sugiyama & Koman, 1979). Chimpanzees of the Taï Forest demonstrate a sophisticated knowledge of the physical properties of objects as they assess up to five physical and contextual properties to select under different circumstances the most optimal hammer for nut-cracking (Boesch & Boesch, 1983, 1984a; Sirianni, Mundry, & Boesch, 2015). This also occurs when out of sight of the food source, demonstrating some foresight and planning (Boesch & Boesch, 1984b). Moreover, it was recently demonstrated that cultural preferences of different neighboring social groups affects the tool selection criteria, emphasizing the multifactorial influences observed in chimpanzee tool use (Luncz & Boesch, 2014; Luncz, Mundry, & Boesch, 2012).
In contrast, captive chimpanzees have demonstrated only limited understanding of how tools work (e.g., Hanus, Mendes, Tennie, & Call, 2011; Martin-Ordas, Call, & Colmenares, 2008; Penn & Povinelli, 2007; Povinelli, 2000; Schrauf & Call, 2011). For example, captive chimpanzees that have spent all their life in an artificial and impoverished captive condition seem to possess limited understanding of the weight of an object and are further challenged when required to combine weight with other properties of a tool (Hanus & Call, 2008; Povinelli, 2012; but see Bril, Dietrich, Foucart, Fuwa, & Hirata, 2009; Schrauf & Call, 2011). Similarly, captive chimpanzees display a limited understanding of the connectivity between objects which prevents them from preferring to pull at a rake or a towel that is in contact with a food reward rather than one that is not (Martin-Ordas et al., 2008; Povinelli, 2000; but see Bania, Harris, Kinsley, & Boysen, 2009).
The most straightforward way to address this question is to compare humans and chimpanzees naturally performing the same technical challenge. In the past, this might have seemed difficult to find but we now know that both chimpanzees and humans, in the African rainforest, open wild-occurring nuts with tools, thereby potentially providing one of the best opportunities to compare the technical skills of two species. The present paper aims to do exactly this.
Here, we assess the technical intelligence hypothesis in the context of a cross-species comparison of nut-cracking in humans and chimpanzees. This is obviously only a sub-sample of the broad repertoire of behaviors exhibited by each species. In addition, our study is limited to examining current behavior, although the behavior has been performed and adapted for generations in both species as evidenced by transport of stone hammers by chimpanzees (Boesch & Boesch, 1984b; Mercader et al., 2007) and use of iron tools by humans. Consequently, our test of the technical intelligence hypothesis restricts itself on the abilities and performances of the two species as seen in the nut-cracking context.
If chimpanzees possess a more limited technical intelligence than humans (e.g., Mithen, 1996; Povinelli, 2000, 2012; Wolpert, 2003), then we would expect humans to use more efficient tools to crack nuts, to modify the tools more extensively, to plan tool use further ahead of time, and to use tools more dexterously to achieve higher efficiencies than chimpanzees. Moreover, higher technical intelligence should allow humans to exploit nut species that are not accessible with less efficient tools. We test these predictions by comparing nut-cracking by the Taï chimpanzees with the same behavior performed by two different human groups; the Aka Pygmy huntergatherers of Central African Republic and the Mbendjele Pygmy hunter-gatherers of the Republic of Congo.
Finally, since cultural effects are known to affect most aspects of human behaviors, we would predict more cultural influences in human technical solutions than in the Taï chimpanzees. One anticipated difference will be that modern hunter-gatherers, and not chimpanzees, are known to use a base camp to where they bring back the gathered foods for consumption and sharing with other group members, rather than consume the opened nuts in the forest as seen in chimpanzees. Limited studies have documented how culture affects technological solution in humans groups and such influences may reinforce or interfere with a pure energetic optimization of nut cracking, as suggested in our previous predictions.
1.1 Background information about nut cracking
1.1.1 The nuts
The nuts found in African forests vary in hardness and morphology and thus present differing technical challenges (Figure 1). For convenience, we use the word “nut” for all hard-shelled fruits found in the forest, although not all of them are nuts in the botanical sense and some should instead be called seeds. Panda oleosa is the hardest nut in Africa and required a weight of about 1,600 kg to break open, while Coula edulis breaks with less than 300 kg and the oil palm nut, Elaeis edulis, is even softer (Boesch & Boesch, 1983; Peters, 1987). For a given nut species, the nut cracker can vary the combination of weight and hardness of the hammer and of the anvil leading to different technical solutions (Figure 1). For example, Panda nuts are too hard to crack open with a wooden hammer, while Coula and Elaeis nuts can be broken with small stones and wooden clubs (Boesch & Boesch, 1983).
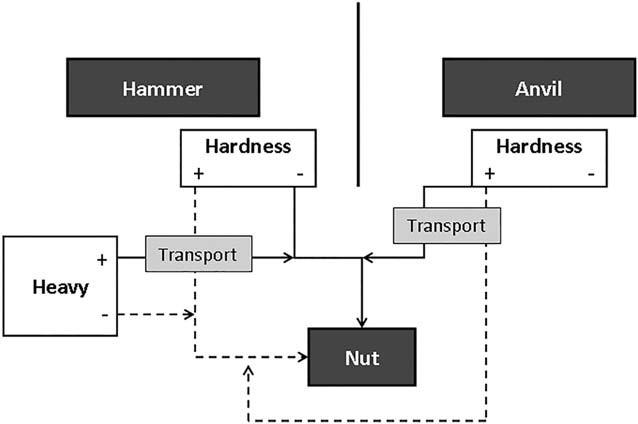
The availability of raw materials for tools, as well as the proximity of trees and tools will influence the technical solutions for the nut crackers. For example, some of the forests in the Congo Basin have few stones and so the cracking of hard nuts, like Panda, requires costly transport of hammers over long distances. Similarly, Panda trees are extremely rare in the forest around Ndele, in Central African Republic, where we found one single tree in a 50 km2 surveyed area, while they are abundant around the village of Djoube, in Congo, where we identified 250 of them in a comparably large area.
In addition to hardness, the morphology of the nuts affects the available technical solutions. C. edulis is a round-shaped nut with one kernel in a thin shell, as is the oil palm nut, Elaeis guinensis. P. oleosa produces oblong-shaped nuts containing 3–4 kernels embedded in a thick hard shell, so that each kernel has to be accessed individually. These three species of nuts can be placed in balance on a flat anvil to be pounded with a hammer. However, Panda nuts need to be positioned carefully to ensure that strikes land between the dehiscent lines of the kernels. In contrast, Irvingia gabonensis, Irvingia robur, or Klainedoxa gabonensis are flat-shaped nuts that germinate along the thin side and need to be held constantly in a specific position to strike them on the dehiscent line.
P. oleosa and E. guinensis nuts are cracked both by humans and chimpanzees, while I. gabonensis (grandifolia) and Klainedoxa gabonica are cracked only by the humans, while the Taï chimpanzee eat their outer juicy pulp when fresh and access the kernel with their teeth once the fruits are drying. Two additional nuts cracked by the human foragers are not found in the Taï forest (I. robur and Antrocarion micraster), while the C. edulis cracked by the Taï chimpanzees is absent in the forests of Central African Republic and Northern Congo. We analyzed nut-cracking for most of these tree species; thus our comparisons of efficiency included nuts cracked by both chimpanzees and humans but also nuts cracked by only one of these primate species.
1.1.2 The nut crackers
1.1.2.1 The Aka of the Central African Republic
The Aka forest hunter-gatherers are part of a larger group of foragers called BaYaka that include the Baka from Cameroon and the Mbendjele from Republic of Congo (see Lewis, 2002; Bahuchet, 1985, 1988, 1991).[5] All subsist largely on wild foods. The BaYaka extract and consume nut kernels throughout much of the year (Bahuchet, 1985, 1988, 1991; Hewlett, Fouts, Boyette, & Hewlett, 2011), while the Aka consume seven nut species depending on their availability: around Badangu, in Lobaye province in the Central African Republic, they rely heavily on oil palm nuts and Irvingia nuts, while farther west around Ndele, where oil palms are absent, they exploit P. oleosa, I. gabonensis (grandifolia), I. robur, K. gabonica, and A. micraster. The density of nut-producing trees in forests in the study regions remains high despite extensive commercial logging in the CAR and northern Congo because their wood is not commercially valuable.
The Aka we followed were in a process of sedentarization in the village of Ndele. The women were still extracting I. gabonensis nuts both for same-day consumption and for production of a bread-like paste that they use in cooking other foods and that lasts up to two or three months. Adam Boyette has over 12 months experience with the Aka and joined C.B. for the data collection period. We followed a group of women for most of the Irvingia nut season during their daily forays in the forest for a 4-week period between May and June 2012. A civil war in 2013 prevented us from returning for the next nut season.
1.1.2.2 The Mbendjele from the Republic of Congo
In the northern Congo, we followed a group of Mbendjele huntergatherers, near the village of Djoube on the Motaba River. The Mbendjele are closely related to the Aka and belong to the same language group (Lewis, 2002, 2012). In this isolated region northwest of the Nouabele-Ndoki National Park, nuts were still an important part of the gathering activities. As with the Aka, CB followed the women in the forest supported by Dasa Bombjakova who has spent more than a year there and was proficient in their language.
TABLE 1 Sample size of the nut-cracking behavior in Taï chimpanzees, Aka and Mbendjele
| Population nut species | Number of adult females | Number of nut-cracking sequences | Total nut-cracking time | Total number of nuts opened |
| Taï chimpanzees | ||||
| Panda oleosa | 18 | 114 | 4,533’ | 3,664 nuts |
| Coula edulis | 22 | 206 | 3,019’ | 7,973 nuts |
| Aka | ||||
| Panda oleosa | 4 | 4 | 28’ | 77 nuts |
| Irvingia gabonensis | 7 | 29 | 693’ | 2,829 nuts |
| Mbendjele | ||||
| Panda oleosa | 17 | 98 | 1,494’ | 2,833 nuts |
| Irvingia gabonensis | 9 | 22 | 286’ | 1,292 nuts |
| Elaeis guineensis | 7 | 53 | 1,670’ | 10,727 nuts |
For each nut species, the number of individual adult females, the number of nut-cracking sequences as well as the total nut-cracking observation time (in minutes) and total number of nuts opened are presented.
This group is more traditional in the sense that it spends more time in temporary camps in the forest and had less tense relations with the farmers living in that region. We followed them in the forest for 12 weeks between May and August 2014, remaining within walking distance to the fallow lands of the villages to allow them regular access to oil palm trees that constitute one of their staple foods. This last aspect might well have been very specific to the region around the Djoube village. The availability of nut-producing trees was higher than in the Ndele forest. During our stay, we gained detailed observations about the Mbendjele extracting nuts of P. oleosa, I. gabonensis, and E. guinensis.
1.1.2.3 The Taï chimpanzees from Cote d^ ’Ivoire
They live in the tropical rainforest of the Taï National Park, in the west of Cote d^ ’Ivoire. For 12 years starting in the early 1980s, Christophe and Hedwige Boesch collected extensive data on nut-cracking behavior (e.g., Boesch, 2012; Boesch & Boesch, 1981, 1983, 1984a, 1984b; Boesch & Boesch-Achermann, 2000). The chimpanzees crack five different species of nuts, including the P. oleosa nuts also cracked by the BaYaka, and the soft C. edulis nuts.
To limit the confounding effects of age and sex we compare only adult female subjects from each species. However, because humans and chimpanzees apply different technical solutions to the same problem, a simple direct comparison was not possible; therefore we will perform two comparisons:
-
Same nuts—different tools: Here, we compare human and chimpanzee nut-cracking technique and performance when opening the hard nuts of P. oleosa. In this comparison, we also include Irvingia nut cracking to compare the Aka and the Mbendjele techniques. Thus, here we explore species- and group-specific differences in technical inventiveness and strategies.
-
Different nuts—same tools: Here we compare humans and chimpanzees using the same tools to open similarly soft nuts. The Mbendjele were cracking the soft oil palm nuts, E. guinensis, while chimpanzees cracked the C. edulis nuts, both species regularly using wooden materials as hammer and anvil. Thus, here we evaluate species-specific technical manipulative skills.
2. Methods
2.1 Data collection procedures
After we explained the reasons for our visit, some women allowed us follow them in order to understand their traditions and how they work. We followed a “neutral-observer non-intervention” approach, in which we followed the women and recorded their behavior without any intervention. Observations were made whenever women went to gather nuts in the forest, and we followed them during the whole foray while keeping track logs of the foray and marking each individual nut-producing tree.
2.1.1 Nut cracking techniques
We used focal individual sampling (Altmann, 1974) to collect data on all the technical elements of the nut cracking (material selected, nut cracking position and technique, tool modification and transport as well as nut cracking efficiency measures) (see Boesch & Boesch, 1981, 1983, 1984a, 1990; Boesch & Boesch-Achermann, 2000). For the Mbendjele, DB and AM collected some data after over one month of training by CB for an attainment of a very high level of concordance in data collection. Later in the season, DB collected all data on oil palm nut cracking by the Mbendjele women (see Table 1). If the target individual stopped cracking nuts before others, we would switch individuals to continue recording nut cracking sequences. Only when the nut cracking was finished did we ask questions about tool selection and the planning of next food source. To increase sample size, we sometimes collected data on two individuals in full view at the same time or switched to another nut cracker if data had already been collected for 30 uninterrupted minutes for one individual under the same tree. Sample sizes are summarized in Table 1.
2.1.2 Efficiency measures
Previous studies of chimpanzee nut-cracking (e.g., Boesch & Boesch, 1984a; Boesch & Boesch-Achermann, 2000) used two measures of efficiency, the number of nuts consumed per minute and the number of hits per edible nut. Because Aka and Mbendjele women generally cooked nuts after cracking them, we modified the first measure to include only the number of nuts opened per minute, given as
Nb nuts open / mn = total time to open all nuts / 5 number of edible nuts
We measured the number of hits per edible nut by summing the total number of hits to all nuts during a given nut-cracking sequence and dividing this by the total number of eaten nuts according to the formula
Nb hits / edible nuts = total number of hits for all nuts / 5 number of edible nuts
This measure is directly influenced by the strategies that nutcrackers use to select material for tools. We used direct observations to obtain these measures for the women. C.B. used archival video footage of Panda nut-cracking to obtain them for the chimpanzees. The video allowed calculation of the total amount of time individuals spent collecting, cracking, and eating all nuts, including those that were inedible. During nut-cracking sessions, the chimpanzees spent 59% of their time eating nuts; thus this measure is not the same as that used in previous studies (above). We calculated both measures only for nutcracking sequences of individuals lasting for more than 5 min. A nutcracking sequence including all observations made of one individual cracking nuts under one and same tree.
2.2 Data analysis and statistical analysis
To compare chimpanzee and Mbendjele nut-cracking efficiencies, we used a Generalized Linear Mixed Model (GLMM; Baayen, 2008) with Poisson error structure and log link function (McCullagh & Nelder, 2008). In the models, we compared nut cracking efficiency (number nuts opened per minute and number hits per nut) between chimpanzees and humans and separately for Panda nuts and soft nuts. In all models, we included group (human or chimpanzee), day in season, and their interaction as fixed effects and individual as a random effect. To keep type I error rate at the nominal level of 0.05, we included random slopes of all fixed effects within individuals (Barr, Levy, Scheepers, & Tily, 2013; Schielzeth & Forstmeier, 2009). However, to keep model complexity moderate, we did not include correlations between random intercepts and random slopes (omitting such correlation does not increase type I error rates; Barr et al., 2013). To control for varying durations of nut cracking sessions we included this variable (log transformed after subtracting the time needed for processing and eating the cracked nuts) as an offset term (McCullagh & Nelder, 2008). For the number of hits per nut model, we did not include an offset term. Since the initial model was overdispersed, we included an additional random effect which had a unique level for each observation (thereafter “observation level random effect”). For the soft nuts the model also included hammer type as a fixed effect (with levels wooden hammer and stone hammer) and random slopes of this effect within individual (with hammer type manually coded). The null models lacked group and its interaction with day in season. Both models included an observation level random effect since otherwise they were overdispersed (dispersion parameter, number nuts: 2.12; hits per nut: 4.33), and the number of nuts model also included the cracking time (log-transformed) as an offset term.
As an overall test of predictors in each model (Forstmeier & Schielzeth, 2011) we compared the full model to a model that did not include the predictor variables, but included all other terms present in the full model. This comparison was conducted using a likelihood ratio test (Dobson, 2002). Prior to fitting the model we z-transformed all fixed effects to a mean of zero and a standard deviation of one. To rule out influential cases we excluded levels of the random effects, one at a time. This revealed the models to be fairly stable. Collinearity, assessed based on Variance Inflation Factors (VIF; Field, 2005; Quinn & Keough, 2002), derived from a standard linear model lacking the respective random effects appeared to be no issue (maximum VIF 5 2.5 in the group comparisons, otherwise <1.03). Overdispersion appeared to be no issue (dispersion parameters, group comparison, number nuts per minute: 0.39; group comparison, number hits per nut: 0.20). The models were fitted in R (version 3.1.2; R Core Team, 2013) using the function glmer of the package lme4 (Bates, Maechler, & Bolker, 2013).
3. Results
Nuts represent for all three groups an important source of food, and during the nut season they were observed to extract nuts for a few hours each day. However, selection of materials and nut-cracking techniques varied extensively among the three groups.

3.1 Material used to crack the nuts
3.1.1 Same nuts—Different tools
The Taï chimpanzees used exclusively natural material to crack the Panda nuts. They showed a high level of selectivity for hard stone material both for the hammers and the anvils, selecting stone as hammer in 89% of the cases (N 5 70 stones/8 sticks) and rock outcrops in 6% of the Panda anvils (N 5 27 rocks/441 roots) despite a very low availability (Boesch & Boesch, 1983) (see Figure 2). When they transported stone hammers for Panda nuts, they selected granite stones, which are relatively rare but especially hard in 88% of cases (401 out of 458 stone hammers, Boesch & Boesch, 1984b). The high proportion of stones used as hammers may result from the fact that using a stone instead of wood allows for an energetic gain of 42% when cracking Panda nuts (Boesch & Boesch, 1983).
In contrast, Aka and Mbendjele women use an axe or a bushknife, respectively, as an anvil to crack open the Panda nuts (see Figures 3 and 4). The Aka place the axe on the ground with the sharp cutting blade edge upwards (Figure 3), and held the nut in place with their hand so that when the nut is hit with a wooden club it will strike the blade precisely at the dehiscent line. In contrast, the Mbendjele mainly used a bushknife as anvil, similarly maintaining the cutting blade upwards on the ground with one foot (Figure 4). Mbendjele women say that axes are too dangerous and are men’s tools, and that they prefer bushknifes, even if we saw them use the axes a few times. We could confirm that they cut their fingers more often with axes than when using a bushknife (although this may be an effect of more extensive practice with bushknives). As hammers they use soft wooden sticks made out of the trunks of small and common saplings (Aka called them “Djele”, and Mbendjele called them “Dofolofo”). Those selected by the Mbendjele are so soft that they required replacement after about 2 hr of use with Panda nuts. Both types of hammers are much lighter and softer than the stones used by the chimpanzees for the same nut species.
Moreover, the Aka and the Mbendjele use a second metal tool, a knife, to extract the kernel from the opened nutshell. After use of the bushknife or axe to cut open the shell precisely along the dehiscent line exposing the whole kernel, the knife is used to extract the kernels intact from the shell to which they remained attached. The extraction of the kernel with a knife is rapidly done and then they throw the kernels in the basket to bring back to camp.

In agreement with the technical intelligence predictions, Aka and Mbendjele women show a more diversified nut spectrum that is not limited to round nuts, as they also crack flat-shaped nuts, like the two species of Irvingia nuts and Klainedoxa nuts. Since those are large trees that produce large numbers of fruits, this expansion in the diet is highly beneficial. Somewhat diverging from the technical intelligence predictions, however, the Aka and Mbendjele showed less selectivity and less flexibility than the chimpanzees in the selection of their tools as a function of the nut hardness, limiting themselves to the same few specialized tools; metal cutting blades as anvil and very similar sized wooden hammers. This more limited tool selection process might reflect the larger cultural dimension of humans in the sense that their tool selection is strongly influenced by the specific usage of their social group. This may still be in agreement with the technical intelligence hypothesis if it leads to higher efficiencies, a test we will perform in the next section. The main cost of using a sharp-cutting anvil instead of a flat one is that it requires holding the nut in position permanently during the strikes, thereby exposing the fingers to cuts if the nut slips along the blade of the anvil.
3.1.2 Different nuts—Same tools
Contrary to what we saw with the hard Panda nuts, Taï chimpanzees and Mbendjele women use very similar materials to crack the softer Coula and Elaeis nuts. Both populations select flat stable anvils on which the nuts are balanced without support; chimpanzees select mostly naturally occurring roots or more rarely rocks (6%), while Mbendjele select a flat stone (40%) when they crack the nuts in the forest, or a wooden mortar, called “kingi leboka” (58%, N 5 32/53) when cracking the nuts in the camp. In both populations, anvils are reused regularly to crack the same nut species across seasons.
As hammers, chimpanzees select mostly small stone hammers (about 80% in South Group chimpanzees; Luncz et al., 2012) or mainly wooden hammers (about 80% in North Group chimpanzees; Luncz et al., 2012). In the group of Mbendjele that we followed, they selected stone hammers in 45% of the nut-cracking sessions in the forest (N 5 24/53) and when in camp used a wooden stick in 21 cases or a metal axe head in 8 cases.
3.2 Nut-cracking technique
3.2.1 Same nuts—Different tools
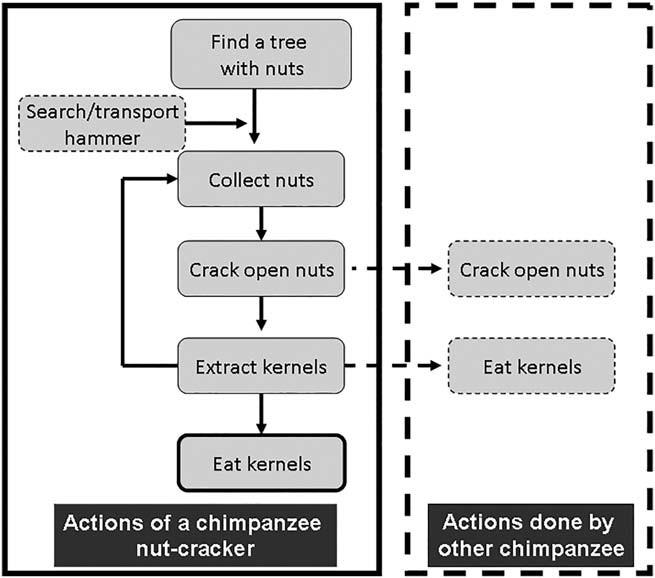
We considered six main elements of the complex nut-cracking process, all of which both the women and the chimpanzees used in the same order and with similar techniques (Figures 5–7). Individuals positioned the nuts precisely on the anvils, stabilized them if necessary, and used a hammer to hit the nut vertically until they cracked.
Taï chimpanzees mainly crack nuts alone, except for females with the dependent offspring (Figures 2 and 5). If no hammer is available at a tree with nuts, chimpanzees select and transport a hammer to crack nuts and they eat all nuts immediately, thus always combining nutcracking with nut-eating for each sequence. Mothers share extensively with their infants and juveniles (Boesch & Boesch-Achermann, 2000). Sharing of nuts with unrelated individuals is exceptionally rare, except in the case of adoption of orphans (Boesch, Bole, Eckhardt, & Boesch, 2010).

The Aka women forage in the forest as groups of four or more individuals which sometimes include men for security (especially when elephants are known to be in the forest). For Irvingia nuts, each woman cracked an average of 226 nuts per tree and an average of 535 nuts per day, which represents about 2.6 kg of nuts per day per woman. Before leaving the camp to collect nuts, the women take in their basket the nut-cracking tools, an axe, a Djele hammer, and a knife. Older experienced women guide the others after they have agreed which trees and/or region to visit. Once under a tree, each woman collects her nuts onto a separate pile and cracks all of them on her own, extracting the kernel with a knife before cracking the next one (Figure 3). Only for especially productive trees will they collect a second pile of nuts to crack. The Aka women both for Irvingia and Panda nuts arrive as a group under the tree, and more than one woman cracks nuts at that tree (Figure 6). Sometimes, a mother opening nuts with an 8–12 year-old daughter may have her help by extracting the kernels with her mother’s knife (Figure 6).
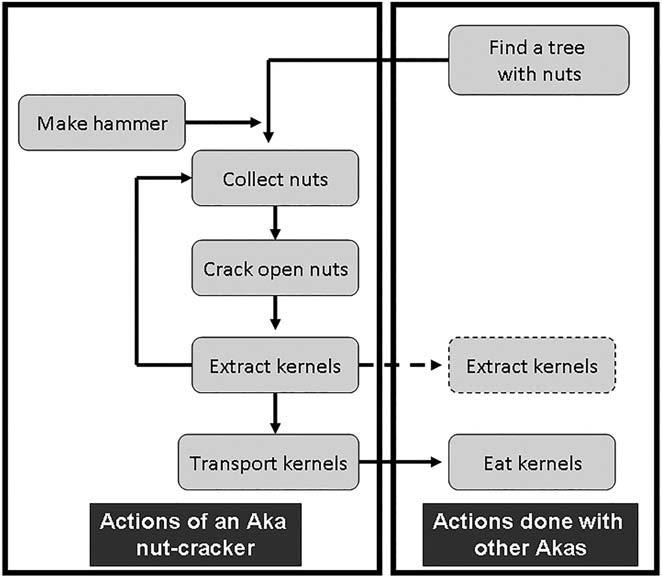
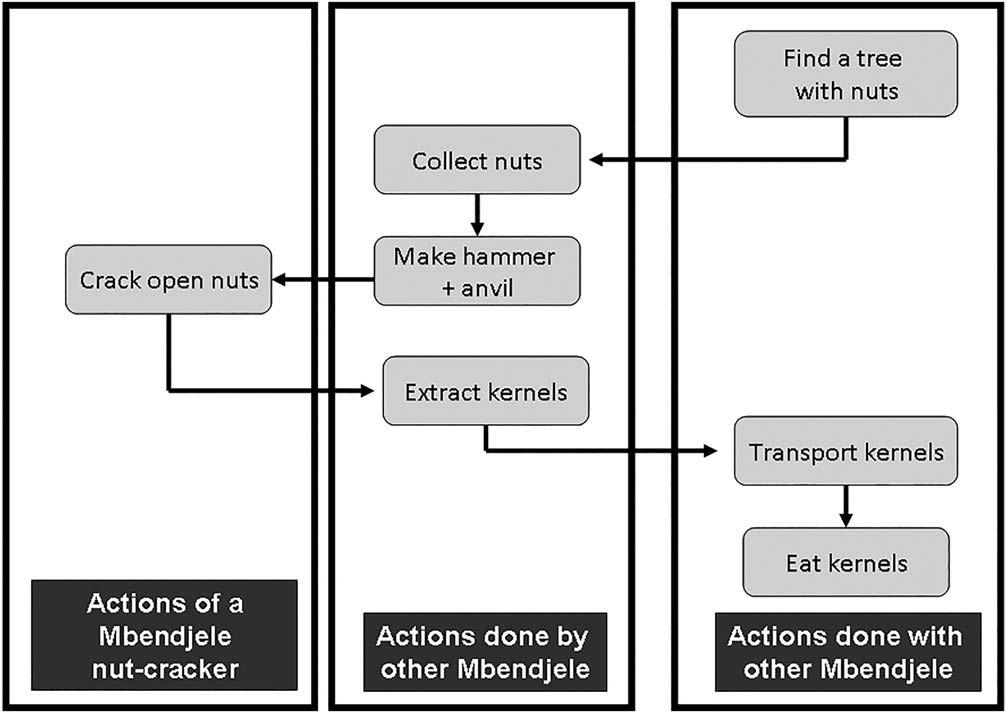
The Mbendjele women have developed more a social approach to nut-cracking, as five of the six elements of the nut-cracking are shared with members of the women group gathering together (Figure 7). Typically, some women collect all of the nuts into one big pile under the same tree and all nut-crackers place themselves in a circle around that pile (Figure 4). Once opened, they place the opened nut parts with one kernel in a pile on a large leaf for the extraction of the kernels to be done by another woman, often the one baby-sitting (Figure 7). Switching between nut-cracking to nut extraction can happen, especially if the baby of a nut-cracking woman wants to breastfeed. Regularly, while collecting nuts, one woman makes some thin wooden hammers for herself and the other women. Cooperative nut collection, making hammers and kernels extraction by Mbendjele women occurred consistently; no such cooperation was observed in chimpanzees.
3.2.2 Different nuts—Same tools
The six main elements found when opening the hard nuts were also observed for the softer Coula nuts in chimpanzees (Figure 5). As for Panda, Coula nut-cracking in chimpanzees is a predominantly individualistic activity with the exception of mother-offspring interactions. Like the chimpanzees, Mbendjele adult women crack palm oil nuts individualistically, both in the forest and in camp. The women also use palm oil as a body lotion, a beauty product and for ritual purposes.
3.3 Efficiency of nut-cracking technique
3.3.1 Same nut—different tools
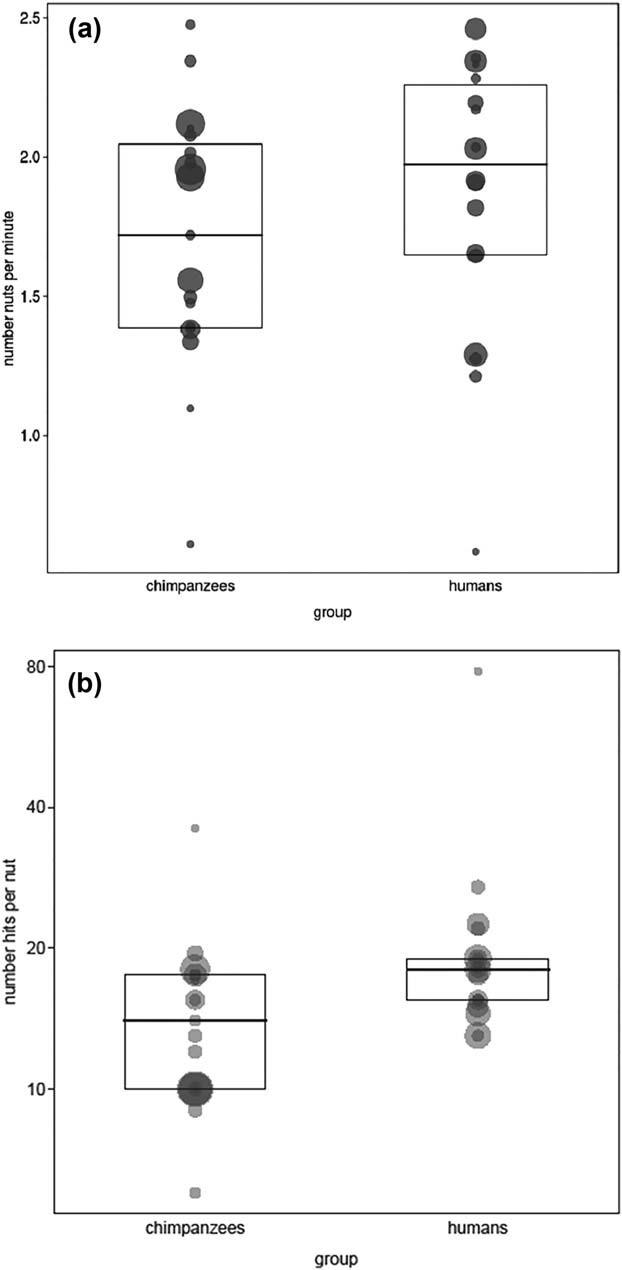
Comparing the number of nuts opened per minute using the two measures presented in the methods allows assessment of how different techniques result in different performances. However, due to the paucity of Panda trees in the forest where the Aka lived, we can compare only Mbendjele efficiencies with those of the Taï chimpanzees. This revealed no obvious difference between the Mbendjele and chimpanzees regarding the first efficiency measure (estimate 6 SE 5 0.06 1 0.08, X25 0.499, df 5 1, p 5 0.480; see also Figure 8a). This is notable as the chimpanzee efficiency is probably underestimated as it is difficult to be certain the measure for chimpanzees was not affected by time spent eating the nuts.
The second efficiency measure, the average number of hits needed to open a nut, was also not significantly different (estimate 6 SE 5 0.016 6 0.132, X25 0.015, p 5 0.904; see also Figure 8b). Chimpanzees use about 40% fewer hits on average, which suggests that their use of stones, which are harder and heavier than the women’s wooden clubs, compensated for any advantages that the cutting anvils provided.
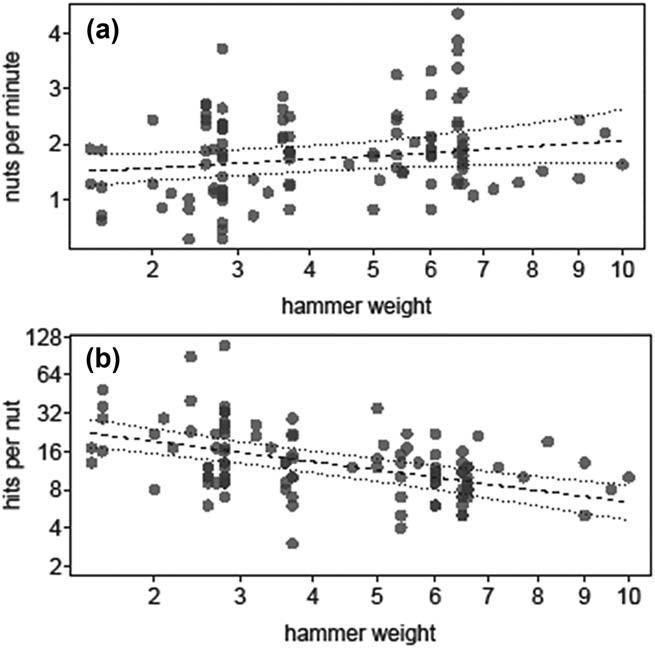
This impression is reinforced when we look at the efficiencies reached by chimpanzees when using hammers of different weights: the heavier the stone hammers used to crack the Panda nuts, the more nuts per minute they tended to open (estimate 6 SE 5 0.076 6 0.041, X2 5 3.073, df 5 1, p 5 0.080) (Figure 9 top) and the fewer hits they needed to open the nuts (estimate 6 SE 5 –0.300 6 0.50, X2 5 17.301, df 5 1, p < 0.001) (Figure 9 below). Chimpanzees have the possibility of selecting a heavier hammer to improve both efficiency measures when cracking Panda nuts, a solution that is not available to the Mbendjele, as they restrict their selection to similar sized wooden clubs.
3.3.2 Different nuts—Same tools
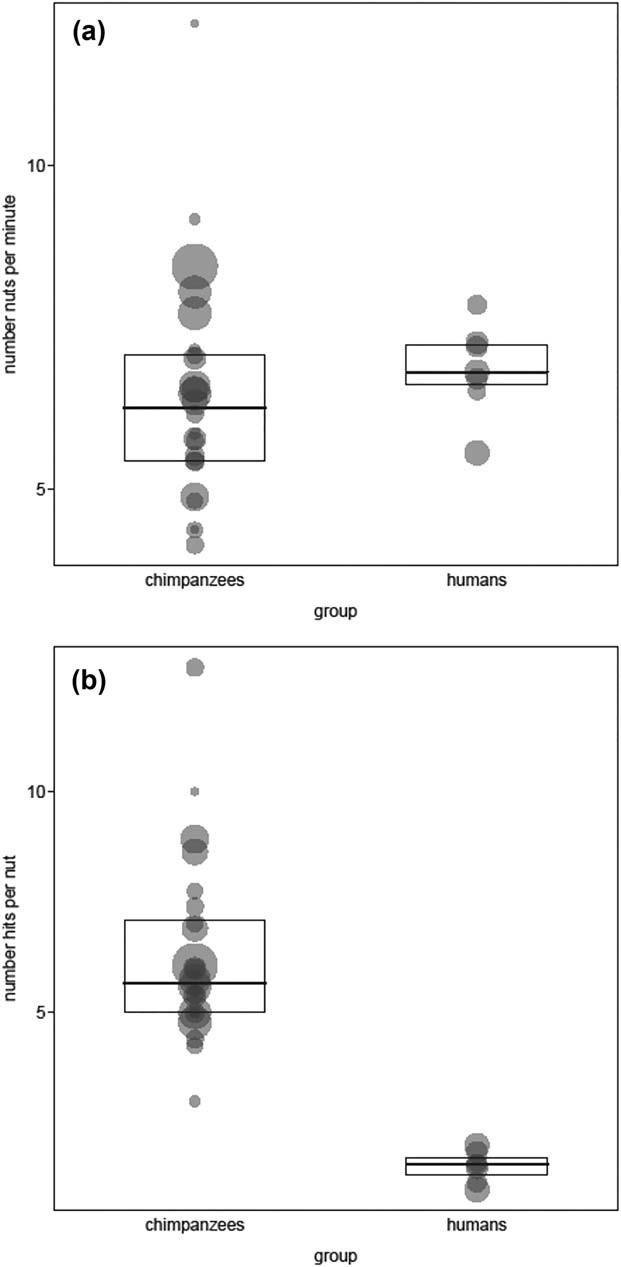
Here with regard to the number of nuts per minute, there was no obvious difference between chimpanzees and Mbendjele efficiencies (estimate 6 SE 5 0.09 6 0.07, X2 5 1.63, df 5 1, p 5 0.202; bootstrapped confidence interval of estimate: 20.06 to 0.24; Figure 10a). However, with regard to the number of hits per nut, humans clearly needed fewer hits than chimpanzees (estimate 6 SE 5 –1.41 1 0.15, X2 5 49.34, df 5 1, p < 0.001; bootstrapped confidence interval: 21.71 to 21.13; Figure 10b).
4. Discussion
According to the technical intelligence hypothesis, humans should possess a more elaborate knowledge of tools and their function, and therefore be better able to solve technical challenges and thereby outperform other animal species, including chimpanzees. The nut-cracking behavior is part of the natural foraging repertoire of both humans and chimpanzees allowing for the first investigation of the technical intelligence hypothesis without the confounds of the arbitrariness and artificiality present in many experimental comparative studies (Bering, 2004; Boesch, 2007; Ferdowsian et al., 2011; Henrich, Heine, & Norenzayan, 2010). Our comparison revealed a large overlap in the solutions adopted by the two species as well as notable differences. Mbendjele women often cooperated during nut-cracking, including by performing complementary tasks, while chimpanzees mostly cracked nuts individualistically, and the women can exploit more types of nuts because they use more specialized tools. However, chimpanzees crack nuts equally or more efficiently by some measures.
Nut cracking presumably appeared very early in human evolution and this percussive behavior is suggested to be at the origin of the invention of the intentional flaking behavior emerging in our ancestors some 2–3.3 million years ago (Bril et al., 2009; Harmand et al., 2015; Nonaka, Bril, & Rein, 2010). Chimpanzee nut-cracking has been the subject of detailed study in West Africa, where the behavior has existed for at least 4,300 years (Boesch & Boesch-Achermann, 2000; Mercader et al., 2007; Mercader, Panger, & Boesch, 2002; Sirianni et al., 2015).
4.1 Contrasting chimpanzee and human nut-cracking technique
Different solutions are available to crack a nut (Figure 1), and chimpanzees concentrate on what is possible with natural tools with surprising success, while humans developed composite artificial tools. In contrast, for soft nuts, humans like chimpanzees choose natural stones as hammers and anvils. Iron tools are a limited resource within the BaYaka society, as there is often only one axe or bushknife per family. Thus, using an axe/bushknife for nut-cracking prevents its use by other family members for other activities such as honey gathering or hunting and in addition imposes transport costs.
The complexity of nut-cracking allows for different technical solutions resulting in comparable benefits. For example, the selection of a specific hammer type can entail different types of benefits. We saw that the Mbendjele select small light hammers while the chimpanzees select heavy hard stone hammers. Both tools have their benefits, with the heavier ones opening nuts with fewer hits and possibly more quickly, while the smaller hammers may provide improved precision when required (such as for Irvingia and Klainedoxa nuts). At the same time, selecting such tools incurs costs that are difficult to compare: a heavy stone needs to be transported by the chimpanzees over distances up to a few hundreds of meters (Boesch & Boesch, 1984b), while the use of a smaller wooden hammer is efficient only if combined with a metal anvil that is transported all day long by the Mbendjele and the Aka women. Another cost comes from the relative softness of tree root anvils which thus absorb a portion of the strike energy. The larger weight of the hammers seems to compensate for this, so that in the end chimpanzees still use fewer hit per nut than Mbendjele women using a bushknife. However, it is important to note that the bushknife is used by women in the forest for many other purposes.
Chimpanzees live in Taï NP in a forest where K. gabonensis and I. gabonensis trees are common and they regularly eat the flesh from fresh fallen frruit. However, they were never seen to use tools to crack them open, although they regularly eat kernels from Irvingia nuts by using their canines to widen small openings that result from germination. This requires considerable force, though, and could lead to tooth breakage. In the Congo forests, the Mbendjele women crack the nuts from K. gabonensis and I. gabonensis as well as the very hard Antrocarion and I. robur nuts. The use of metal tools thus allows the exploitation of many more of the abundant hard nuts available in those forests. Chimpanzees are strongly limited to those hard nuts that can be balanced on a flat anvil.
On the other hand, use of sharp-edged metal cutting tools may entail some costs, especially when used to open hard-shelled nuts. Both the axe used by the Aka women and the bushknife used by the Mbendjele produced occasional cuts to the fingers that resulted in bleeding and sometimes caused even deeper cuts that prevented them from cracking nuts for a few days. This especially occurred for younger individuals learning the technique and in a subsequent analysis we will study how such a risk affects the learning of the technique.
4.2 Technical intelligence and nut cracking
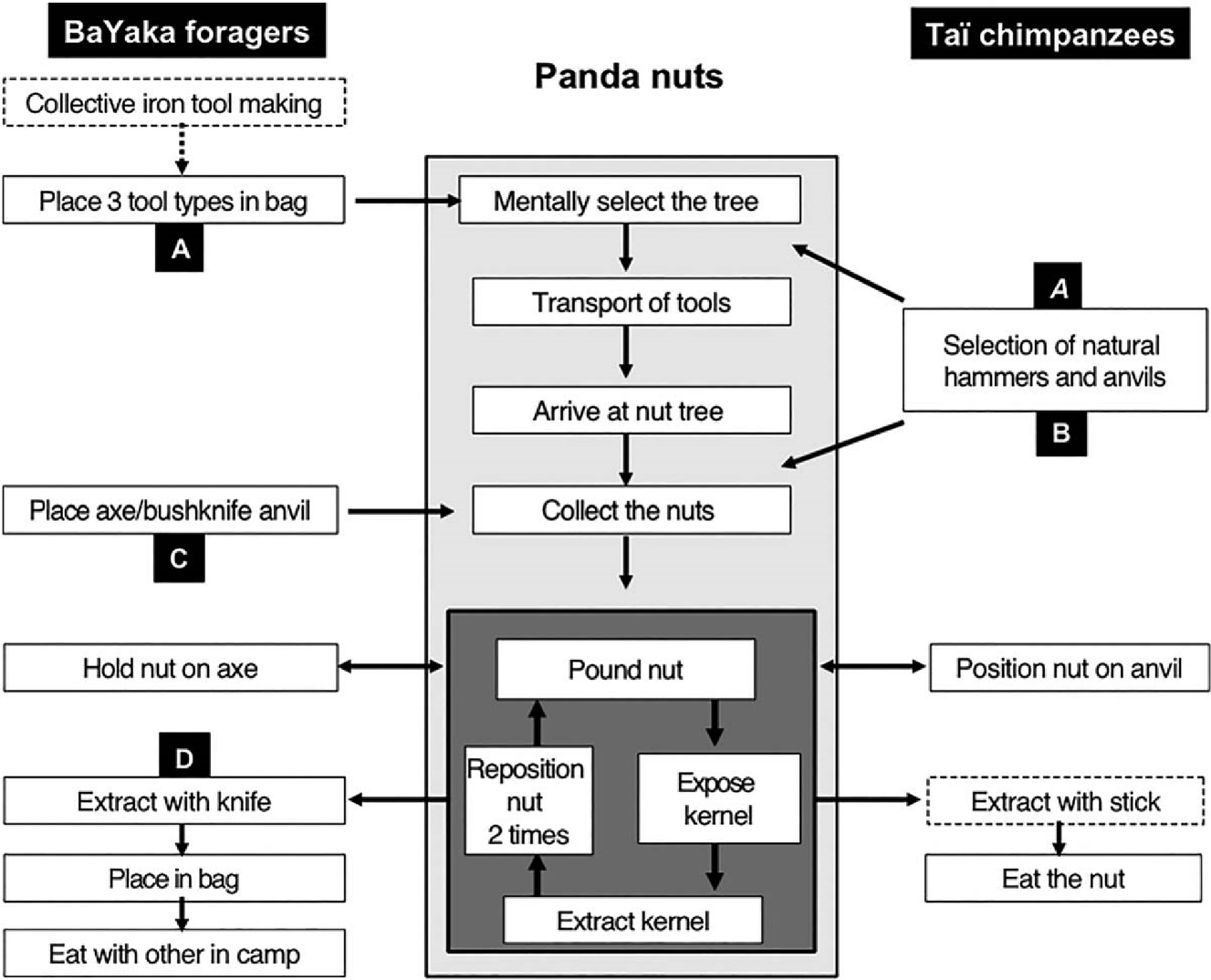
Which aspects of technical intelligence may explain the differences we observed between humans and chimpanzees when cracking nuts? Figure 11 lists the four main cognitive skills we see at work within the nut-cracking sequence that can explain part of the differences discussed above.
-
Planning of actions: Some have proposed this to be one of the distinguishing cognitive abilities between humans and other animals (Cheke & Clayton, 2010; Roberts, 2002; Suddendorf & Corballis, 2007, but see Janmaat, Ban, & Boesch, 2013a, 2013b). For the hard Panda nuts, both species plan by anticipating the need of a specific hammer/anvil and transport it if needed to the nut cracking tree (see Figure 11). By using both an artificial anvil and commonly occurring wood as a hammer, Mbendjele women have partly freed themselves from the limitations of material availability in the forest. For chimpanzees, nut-cracking may be energetically unfeasible in forests of limited material availability, while the Mbendjele women could still crack the nuts under such conditions. For example, large granite stones, commonly used by chimpanzees for Panda nuts, are extremely rare in the Aka and Mbendjele forests but this does not affect their performance. This difference between the two species points to a clear selective advantage for action planning.
-
Conditional selection of tools: In a recent analysis, we showed thatthe hammer selection process for Coula nut-cracking by Taï chimpanzees is highly complex, and included simultaneous assessment of four different parameters of the tool and hammer selection dependent upon the local material available (Sirianni et al., 2015). An earlier, less detailed study tended to support a very similar awareness of at least three functional properties of stone hammers for Panda nut-cracking in Taï chimpanzees (Boesch & Boesch, 1984b). Nut cracking represents one of the most challenging high benefit foraging behaviors seen in nature and chimpanzees have developed sophisticated cognitive skills to achieve a net benefit despite important environmental constraints (Milton, 1988). In contrast, humans have partly freed themselves from such environmental constraints and followed a comparatively simple and rigid selection strategy by always using the same anvil and selecting hammers among a very limited number of common sapling species. This is clearly less complex than what we see in chimpanzees and is unexpected under the technical intelligence hypothesis. In addition, it entails costs as the efficiency measures for hard nuts tend to be lower for humans compared to chimpanzees (see Figure 8b). Thus, on one side and when comparing the same nut species, chimpanzees demonstrate a more elaborate selection of tools than humans. On the other side, the potential limitation in the human’s selection flexibility is largely compensated by a larger access to more nut species.
It is further noteworthy that chimpanzees crack Coula nuts at the beginning of the season directly in the branches of the nutproducing trees by selecting anvils that can sometimes be far from horizontal (Figure 2). In those situations, chimpanzees hold the nut in place on the anvil with one hand while pounding it with the hammer held in the other hand (Boesch & Boesch, 1984a, 1984b). To avoid hitting their fingers during striking the nuts, they swiftly release the grip on the nut, holding it back firmly before lifting the hammer (Boesch & Boesch, 1990). This tree-technique uniquely seen in chimpanzees allows them to expand the nut-cracking season by a whole month and therefore maximize the amount of nuts they can consume.
-
Composite tools: After many decades of observations on wild chimpanzees living in different environments, we have yet to find a population able to produce composite tools or sharp-edge tools (Ambrose, 2010; Boesch, 2012), suggesting a striking limitation in the technical abilities of chimpanzees as compared to humans. Unintentional flake production happens regularly when chimpanzees use stones to crack nuts, as has been documented in an archeological study in the Taï forest (Mercader et al., 2002) as well as directly observed (Boesch, personal observation). Nevertheless, chimpanzees have never been seen to use the sharp-edge of such flakes, even while they reuse them as smaller hammer to crack nuts with the same pounding technique. Chimpanzees have been seen to use tools in a sequential order that can include tools of different materials (Boesch, 2012; Boesch, Head, & Robbins, 2009), but they have never been seen to combine them into one composite tool. This supports a striking difference in technical intelligence between chimpanzees and humans.
Present-day composite tools include the metal tools which were introduced in Central Africa only recently (Bahuchet, 1991, and Lupo et al., 2015 suggest iron introduction and production possibly as late as AD 1787), which raises the question of what type of composite tools were the BaYaka using to crack nuts before the introduction of iron ore? Were they using the same technique with stone handaxes? It is, however, still unclear how resistant these would have been for cracking the hard Panda nuts (Hayden, 2008, 2015). So it might be that, as they still do with soft nuts, the BaYaka people could have used a more chimpanzee-like technique before the Iron Age. For example, when cracking only a few nuts, Mbendjele women may use roots as an anvil, as chimpanzees do, and place the Panda nuts on it and use the bushknife as a hammer (a technique regularly seen in the present-day Baka of Cameroon; Sato, Kawamura, Hayashi, Inai, & Yamauchi, 2012).
Finally, the evolution of composite tools should be expected only in situations where it brings some selective advantage. This is not the case for Panda nut-cracking technique where simple tools are equally efficient (see Figure 8), so the inclusion of composite tools in the nut-cracking technique needs to be explained. One possibility is that in the BaYaka society bushknifes and axes are multi-function tools, and while now an important element for cracking nuts, they may originally have been used to cut firewood, to cut the large Treculia fruits to access the prized numerous seeds, to dig out wild yam tubers, to cut new wooden hammers and so on. Once these benefits of bushknifes were established they may have been employed in the nut-cracking technique.
-
Delayed food sharing: The notion of Home Base has been central inour understanding of some of the key innovations leading to modern humans (Isaac, 1978; Kaplan, Hill, Lancaster, & Hurtado, 2000; Marlowe, 2005). In all traditional hunter-gatherer societies, part of the food collected in the field is brought back to camp for sharing with individuals that were not present during collection because they were foraging for other food sources or did not forage at all. Our observations of nut-cracking in the forest by the BaYaka women concur with this view and stand in strong contrast with the chimpanzee habit of consuming everything where the food has been accessed. Food sharing has been amply documented in chimpanzees for meat (Boesch & Boesch, 1989; Gilby, 2006; Teleki, 1973; Watts & Mitani, 2002), for nuts (Boesch & BoeschAchermann, 2000), and for some large fruits (Gomes & Boesch, 2011; Wittig & Boesch, 2003), however the sharing happens always at the time of consumption and is not delayed in the way it is in humans.
Food sharing in camps in humans has been proposed to fulfill a very important social function and play a central role in the development of a division of labor between the sexes and among different age classes (Isaac, 1978; Marlowe, 2005; Winterhalder, 1997; Winterhalder & Smith, 2000). This social function is partly observed in chimpanzees where food, especially meat, can be shared strategically and traded with social partners (Boesch & Boesch, 1989; Gomes & Boesch, 2009, 2011; Mitani, Watts, & Muller, 2002).
4.3 Socio-cultural aspects of technical solutions
The technical complexity of nut-cracking and the varying availability of potential tools results in individuals adopting different solutions that may be equally efficient (Figure 1). This flexibility provides room for different technical solutions in different groups. For example, in Taï chimpanzees, different neighboring groups have developed different preferences for the material of the hammers they use when cracking the Coula nuts and individuals within each social group show a very high level of conformity to their group’s solution (Luncz et al., 2012, 2014; Luncz, Wittig, & Boesch, 2015). Furthermore, these different material preferences were only in part affected by material availability in the different group territory, supporting clearly a cultural influence on material selection (Luncz et al., 2012). Similarly, anvil selection differs in the two BaYaka groups we compared, with the Aka using only axes and the Mbendjele using mainly bushknives. This difference seems to come at a cost, as the Mbendjele women are significatively less efficient than the Aka women when cracking the same I. gabonensis nuts (called “Mopayo” or “Payo” respectively; Boesch, unpublished data). Axes have greater cutting power than bushknives, and once their use is mastered they allow the opening of very hard nuts more efficiently than bushknives. Finally, Mbendjele women select heavier branches as hammers for Irvingia nuts than Aka women. This may be viewed as a way to compensate for the lower cutting power of the bushknifes (see Figure 1), but this is complicated by the fact that Mbendjele open the nuts “from the head,”[6] while Aka women open them from the base to make the extraction of the kernel easier.
The largest difference in nut-cracking as a social activity was the extensive cooperation among Aka and, especially, Mbendjele women, in contrast to the individualistic nut-cracking by chimpanzees. Thus, we see here a case of “facultative cooperation” as it reflects more the social cohesion within group members than an adaptive response to an ecological challenge (West et al., 2006).
An intriguing question is why we see a difference in the level of cooperation between Aka and Mbendjele women. At least two possible factors may explain this. First, Aka women foraging around the larger village of Ndele faced relatively higher levels of feeding competition. We regularly observed that Aka women might arrive at fruiting Irvingia trees only to find that other women are already cracking the nuts or that all nuts had been collected by others since their last visit. Such situations never happened when we were with the Mbendjele where Panda trees are very abundant and the women could easily fill their baskets close to camp and this may have allowed their higher levels of cooperation. A second factor may be differences in the level of sedentarization. Lewis, Vinicius, Strods, Mace, and Migliano (2014) proposed that extensive cooperation in hunter-gatherer societies resulted from a combination of high mobility with prevalent demand sharing, and further argued that it could persist without punishment of noncooperator. However, they added that cooperation becomes less likely as groups became less mobile. This might help to explain why we found less complex cooperation among the Aka, who were in the process of sedentarization, and the Mbendjele, who relied much more on temporary camps and were more mobile.
5. Conclusion
A direct comparison of nut cracking between chimpanzees and humans in the context of the technical intelligence hypothesis has revealed a complex picture. In some aspects humans performed in more complex and efficient ways than chimpanzees, as predicted, while in other aspects chimpanzees outperformed humans. This revealed as well that both species were able to find different and efficient technical solutions to the challenge of opening hard nuts, with chimpanzees relying more on flexible solutions reevaluated for each nut-cracking situation, while humans rely systematically on the same high performance tools in all different nut-cracking situations. The more flexible solutions adopted in chimpanzees rewarded them with better performance in some measurements. However, the more socially integrated human solutions allow for the complementary work of different individuals to crack the nuts. Cooperation levels differed in the two humans groups for aspects of the nut-cracking that one individual could master on its own. Chimpanzee and human each have sophisticated technical intelligence skills to solve in their own ways the complex challenges of cracking hard nuts with the help of tools.
Acknowledgments
We thank the authorities from the Central African Republic and the Republic of Congo for granting us all necessary permissions to work in their country and especially thank Timothee Tikouzou in Bangui and Prof. Clobite Bouka Biona in Brazzaville for logistical support. We thank the Max Planck Society for continuous financial support. This study was approved by the Ethic Committee of the Max Planck Society. Barry Hewlett and Jerome Lewis were instrumental for making the study possible with the Aka and Mbendjele foraging people, Roger Mundry provided essential support for the statistical analysis of the nut-cracking efficiency data, Linda Vigilant and an anonymous reviewer provided much needed assistance in editing the English, and all receive our thanks. We are especially grateful to the people in the Aka village of Ndele as well as those from the Mbendjele village of Djoube welcoming us so wholeheartedly, being so tolerant of our presence on their daily forays in the forest and being so open in sharing their traditions with us during our stays with them. Without them our study would not have been possible.
References
Altmann, J. (1974). Observational study of behaviour: Sampling methods.
Behaviour, 49, 227–265.
Ambrose, S. (2010). Coevolution of composite-tool technology, constructive memory and language. Current Anthropology, 51, S135. S141.
Baayen, H. (2008). Analyzing linguistic data. Cambridge: Cambridge University Press.
Bahuchet, S. (1985). Les pygmees aka et la for^et centrafricaine. Paris: Selaf, CNRS.
Bahuchet, S. (1988). Food supply uncertainty among the Aka pygmies (Lobaye, Central African Republic). In I. Garine & G. Harrison (Eds.), Coping with uncertainty in food supply (pp. 118–149). Oxford: Oxford University Press.
Bahuchet, S. (1991). Spatial mobility and access to resources among African pygmies. In M. Casimir & A. Rao (Eds.), Mobility and territoriality: Social and spatial boundaries among foragers, fishers, pastoraliststs (pp. 205–257). Oxford: Berg Publishers.
Bania, A., Harris, S., Kinsley, H., & Boysen, S. (2009). Constructive and deconstructive tool modification by chimpanzees (Pan troglodytes). Animal Cognition, 12, 85–95.
Bar-Yosef, O., & van Peer, P. (2009). The chaine operatoire approach in the Middle Paleolithic archaeology. Current Anthropology, 50, 103– 117.
Barr, D., Levy, R., Scheepers, C., & Tily, H. (2013). Random effects structure for confirmatory hypotheis testing: Keep it maximal. Journal of Memory and Language, 68, 255–278.
Bates, D., Maechler, M., & Bolker, B. (2013). lme4: Linear mixed-effects models using S4 classes. R package version 0.999999–2.
Beaune, S. (2004). The invention of technology: Prehistory and cognition. Current Anthropology, 45, 139–151.
Bering, J. (2004). A critical review of the “enculturation hypothesis”: The effects of human rearing on great ape social cognition. Animal Cognition, 7, 201–212.
Boesch, C. (2007). What makes us human (Homo sapiens)? The challenge of cognitive cross-species comparison. Journal of Comparative Psychology, 121, 227–240.
Boesch, C. (2012). Wild cultures: A comparison between chimpanzee and human cultures. Cambridge: Cambridge University Press.
Boesch, C., & Boesch, H. (1981). Sex differences in the use of natural hammers by wild chimpanzees: A preliminary report. Journal of Human Evolution, 10, 585–593.
Boesch, C., & Boesch, H. (1983). Optimization of nut-cracking with natural hammers by wild chimpanzees. Behaviour, 83, 265–286.
Boesch, C., & Boesch, H. (1984a). Possible causes of sex differences in the use of natural hammers by wild chimpanzees. Journal of Human Evolution, 13, 415–440.
Boesch, C., & Boesch, H. (1984b). Mental map in wild chimpanzees: An analysis of hammer transports for nut cracking. Primates, 25, 160– 170.
Boesch, C., & Boesch, H. (1989). Hunting behavior of wild chimpanzees in the Taï National Park. American Journal of Physical Anthropology, 78, 547–573.
Boesch, C., & Boesch, H. (1990). Tool use and tool making in wild chimpanzees. Folia Primatologica, 54, 86–99.
Boesch, C., & Boesch-Achermann, H. (2000). The chimpanzees of the Taï forest: Behavioural ecology and evolution. Oxford: Oxford University Press.
Boesch, C., Bole, C., Eckhardt, N., & Boesch, H. (2010). Altruism in chimpanzees: The case of adoption. PLoS ONE, 5, e8901.
Boesch, C., Head, J., & Robbins, M. (2009). Complex toolsets for honey extraction among chimpanzees in Loango National Park, Gabon. Journal of Human Evolution, 56, 560–569.
Bril, B., Dietrich, G., Foucart, J., Fuwa, K., & Hirata, S. (2009). Tool use as a way to assess cognition: How do captive chimpanzees handle the weight of the hammer when cracking a nut? Animal Cognition, 12, 217–235.
Cheke, L., & Clayton, N. (2010). Mental time travel in animals. Wiley Interdisciplinary Reviews: Cognitive Science, 1, 915–930.
Davidson, I., & Noble, W. (1993). Tools and language in human evolution. In K. Gibson & t. Ingold (Eds.), Tools, language and intelligence: Evolutionary implications (pp. 363–388). Cambridge: Cambridge University Press.
Dietrich, S., Toth, N., Schick, K., & Chaminade, T. (2008). Neural correlates of early Stone Age toolmaking: Technology, language and cognition in human evolution. Philosophical Transactions of the Royal Society B, 363, 1939–1949.
Ferdowsian, H., Durham, D., Kimwele, C., Kranendonk, G., Otali, E., Akugizibwe, T., ... Johnson, C. (2011). Signs of mood and anxiety disorders in chimpanzees. PLoS ONE, 6, e19855.
Field, A. (2005). Discovering statistics using SPSS. London: Sage Publications.
Foley, R., & Lahr, M. (2003). On stony ground: Lithic technology, human evolution, and the emergence of culture. Evolutionary Anthropology, 12, 109–122.
Forstmeier, W., & Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behavioral Ecology and Sociobiology, 65, 47–55.
Gilby, I. (2006). Meat sharing among the Gombe chimpanzees: Harassment and reciprocal exchange. Animal Behavior, 71, 953–963.
Gomes, C., & Boesch, C. (2009). Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE, 4, e5116.
Gomes, C., & Boesch, C. (2011). Reciprocity and trades in wild West African chimpanzees. Behavioral Ecology and Sociobiology, 65, 2183–2196.
Goodall, J. (1970). Tool-using in primates and other vertebrates. In D. Lehrmann, R. Hinde, & E. Shaw (Eds.), Advances in the study of behavior (Vol. 3, pp. 195–249). New York: Academic Press, New York.
Goodall, J. (1986). The chimpanzees of Gombe: Patterns of behavior. Cambridge: The Belknap Press of Havard University Press.
Hanus, D., & Call, J. (2008). Chimpanzees infer the location of a reward on the basis of the effect of its weight. Current Biology, 18, R370–R372.
Hanus, B., Mendes, N., Tennie, C., & Call, J. (2011). Comparing the performances of apes (Gorilla gorilla, Pan troglodytes, Pongo pygmaeus) and human children (Homo sapiens) in the floating peanut task. PLoS ONE, 6, e19555.
Harmand, S., Lewis, J., Feible, C., Lepre, C., Prat, S., Lenoble, A., ... Roche, H. (2015). 3.3-Million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature, 521, 310–316.
Hayden, B. (2008). What were they doing in the Oldowan? Lithic Technology, 33, 105–139.
Hayden, B. (2015). Insights into early lithic technologies from ethnography. Philosophical Transactions of the Royal Society B, 370, 20140356.
Henrich, J., Heine, S., & Norenzayan, A. (2010). The weirdest people in the world? Behavioral and Brain Sciences, 33, 61–135.
Henshilwood, C., D’errico, F., Marean, C., Milo, R., & Yates, R. (2001). An early bone tool industry from the Middle Stone Age at Blombos Cave, South Africa: Implications for the origins of modern human behaviour, symbolism and language. Journal of Human Evolution, 41, 631–678.
Hewlett, B., Fouts, H., Boyette, A., & Hewlett, B. (2011). Social learning among Congo Basin hunter-gatherers. Philosophical Transactions of the Royal Society B, 366, 1168–1178.
Iovita, R., & McPherron, S. (2011). The handaxe reloaded: A morphometric reassessment of Acheuliand and Middle Paleolithic handaxes. Journal of Human Evolution, 61, 61–74.
Isaac, G. (1978). The food sharing behavior of protohuman hominids. Scientific American, 238, 90–108.
Janmaat, K., Ban, S., & Boesch, C. (2013a). Tai chimpanzees use botanical skills to discover fruit: What we can learn from their mistakes. Animal Cognition, 16, 851–860.
Janmaat, K., Ban, S., & Boesch, C. (2013b). Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Animal Behavior, 86, 1183–1205.
Johnson-Frey, S. (2003). What’s so special about human tool use? Neuron, 39, 201–204.
Kaplan, H., Hill, K., Lancaster, J., & Hurtado, A. (2000). A theory of human life history evolution: Diet, intelligence and longevity. Evolutionary Anthropology, 9, 156–185.
Leakey, R. (1980). The making of mankind. London: Book Club Associates.
Lemorini, C., Plummer, T., Braun, D., Crittenden, A., Ditchfield, P., Bishop, L., ... Potts, R. (2014). Old stones’ song: Use-wear experiments and analysis of the Oldowan quartz and quartzite assemblage from Kanjera South (Kenya). Journal of Human Evolution, 72, 10–25.
Lewis, J. (2002). Forest hunter-gatherers and their world: A study of the Mbendjele Yaka Pygmies and their secular and religious activities and representations (PhD thesis). London School of Economics and Political Science, University of London.
Lewis, J. (2012). Technological leap-frogging in the Congo Basin, Pygmies and global positioning systems in central Africa: What has happened and where is it going? African Study Monographs Supplement, 43, 15–44.
Lewis, H., Vinicius, L., Strods, J., Mace, R., & Migliano, A. (2014). High mobility explains demand sharing and enforced cooperation in egalitarian hunter-gatherers. Nature Communications, 5, 5789. doi: 10.1038/ncomms6789
Luncz, L., & Boesch, C. (2014). Tradition over trend: Neighboring chimpanzee communities maintain differences in cultural behavior despite frequent immigration of adult females. American Journal of Primatology, 76, 649–657.
Luncz, L., Mundry, R., & Boesch, C. (2012). Evidence for cultural differences between neighboring chimpanzee communities. Current Biology, 22, 922–926.
Luncz, L., Wittig, R., & Boesch, C. (2015). Primate archaeology reveals cultural transmission in wild chimpanzees (Pan troglodytes verus). Philosophical Transactions of the Royal Society B, 370, 21040348.
Lupo, K., Schmitt, D., Kiahtipes, C., Ndanga, J., Young, C., & Simiti, B. (2015). On intensive Late Holocene iron mining and production in the Northern Congo Basin and the environmental consequences associated with metallurgy in Central Africa. PLoS ONE, 10, e0132632.
Marlowe, F. (2005). Hunter-gatherers and human evolution. Evolutionary Anthropology, 14, 54–67.
Martin-Ordas, G., Call, J., & Colmenares, F. (2008). Tubes, tables and traps: Great apes solve two functionally equivalent trap tasks but show no evidence of transfer across tasks. Animal Cognition, 11, 423–430.
McBrearty, S., & Brooks, A. (2000). The revolution that wasn’t: A new interpretation of the origin of modern human behavior. Journal of Human Evolution, 29, 453–563.
McCullagh, P., & Nelder, J. (2008). Generalized linear models. London: Chapman and Hall.
McPherron, S. (2000). Handaxes as a measure of the mental capabilities of early hominids. Journal of Archaeological Science, 27, 655–663.
McPherron, S. (2013). Perspectives on stone tools and cognition in the early Paleolithic record. In C. Sanz, J. Call, & C. Boesch (Eds.), Tool use in animals: Cognition and ecology (pp. 286–309). Cambridge: Cambridge University Press.
Mellars, P., & Stringer, C. (1989). The human revolution: Behavioural and biological perspectives on the origins of modern humans. Edinburgh: Edinburgh University Press.
Mercader, J., Barton, H., Gillespie, J., Harris, J., Kuhn, S., Tyler, R., & Boesch, C. (2007). 4,300-year-old chimpanzee sites and the origins of percussive stone technology. Proceedings of the National Academy of Sciences of the United States of America, 104, 3043–3048.
Mercader, J., Panger, M., & Boesch, C. (2002). Excavation of a chimpanzee stone tool site in the African rainforest. Science, 296, 1452– 1455.
Milton, K. (1988). Foraging behaviour and the evolution of primate intelligence. In R. W. Byrne & A. Whiten (Eds.), Machiavellian intelligence: Social expertise and the evolution of intellect in monkeys, apes and humans (pp. 285–305). Oxford: Clarendon Press.
Mitani, J., Watts, D., & Muller, M. (2002). Recent development in the study of wild chimpanzee behaviour. Evolutionary Anthropology, 11, 9–25.
Mithen, S. (1996). The prehistory of mind: The cognitive origin of art and science. London: Thames and Hudson.
Nonaka, T., Bril, B., & Rein, R. (2010). How do stone knappers predict and control the outcome of flaking? Implications for understanding early stone tool technology. Journal of Human Evolution, 59, 155–167.
Oakley, K. (1956). Man the tool-maker. London: British Museum.
Penn, D., & Povinelli, D. (2007). On the lack of evidence that non-human animals possess anything remotely resembling a “theory of mind”.
Philosophical Transactions of the Royal Society B, 362, 731–744.
Peters, C. (1987). Nut-like oil seeds: Food for monkeys, chimpanzees, humans and probably ape-men. American Journal of Physical Anthropology, 73, 333–363.
Povinelli, D. (2000). Folk physics for apes: The chimpanzee’s theory of how the world works. Oxford: Oxford University Press.
Povinelli, D. (2012). World without weight: Perspectives on an alien mind. Oxford: Oxford University Press.
Quinn, G., & Keough, M. (2002). Experimental designs, confidence interval and statistical significance: A practical guide for biologists. Biological Reviews, 82, 591–605.
R Core Team. (2013). R: A language and environment for statistical computing. R foundation for statistical computing. Austria, Vienna.
Roberts, W. (2002). Are animals stuck in time? Psychological Bulletin, 128, 473–489.
Sanz, C., & Morgan, D. (2007). Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. Journal of Human Evolution, 52, 420–433.
Sanz, C., & Morgan, D. (2009). Flexible and persistent tool-using strategies in honey-gathering by wild chimpanzees. International Journal of Primatology, 30, 411–427.
Sato, H., Kawamura, K., Hayashi, K., Inai, H., & Yamauchi, T. (2012). Addressing the wild yam question: How Baka hunter-gatherers acted and lived during two controlled foraging trips in the tropical rainforest of southeastern Cameroon. Anthropological Science, 120, 129–149.
Schrauf, C., & Call, J. (2011). Great apes use weight as a cue to find hidden food. American Journal of Primatology, 73, 323–334.
Schielzeth, H., & Forstmeier, W. (2009). Conclusions beyond support: Overconfident estimates in mixed models. Behavioral Ecology, 20, 416–420.
Sirianni, G., Mundry, R., & Boesch, C. (2015). When to choose which tool: Multidimensional and conditional selection of nut-cracking hammers in wild chimpanzees. Animal Behavior, 100, 152–165.
Suddendorf, T., & Corballis, M. (2007). The evolution of foresight: What is mental travel and is it unique to humans? Behavioral and Brain Sciences, 30, 299–351.
Sugiyama, Y., & Koman, J. (1979). Tool-using and -making behavior in wild chimpanzees at Bossou, Guinea. Primates, 20, 513–524.
Teleki, G. (1973). The predatory behavior of wild chimpanzees. Brunswick: Bucknell University Press.
Watts, D., & Mitani, J. (2002). Hunting and meat sharing by chimpanzees ot Ngogo, Kibale National Park, Uganda. In C. Boesch, G. Hohmann, & L. Marchant (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 244–255). Cambridge: Cambridge University Press.
Wittig, R., & Boesch, C. (2003). Food competition and linear dominance hierarchy among female Pan troglodytes verus of the Taï National Park. International Journal of Primatology, 24, 847–867.
West, S., Gardner, A., Shuker, D., Reynolds, T., Burton-Chellow, M., Sykes, E., ... Griffin, A. (2006). Cooperation and the scale of competition in humans. Current Biology, 16, 1103–1106.
Winterhalder, B. (1997). Social foraging and the behavioral ecology of introgroup resource transfers. Evolutionary Anthropology, 5, 46–57.
Winterhalder, B., & Smith, E. (2000). Analyzing adaptive strategies: Human baheavioral ecology at twenty-five. Evolutionary Anthropology, 5:51–72.
Wolpert, L. (2003). Causal belief and the origins of technology. Philosophical Transactions of the Royal Society of London A, 361, 1709–1719.
Wynn, T. (1993). Layers of thinking in tool behavior. In T. Ingold & K. Gibson (Eds.), Tools, language and intelligence: Evolutionary implications (pp. 389–406). Cambridge: Cambridge University Press.
Wynn, T. (2002). Archeology and cognitive evolution. Behavioral and Brain Sciences, 25, 389–438.
[1] Department of Primatology, Max Planck Institute of Evolutionary Anthropology, Leipzig, Germany
[2] Department of Social Anthropology,
University College London, London, United Kingdom
[3] Thompson writing program, University of Duke, Durham
[4] Department of Primatology, Max Planck Institute of Evolutionary Anthropology, Leipzig, Germany
[5] The word “Pygmy” has gained a pejorative connotation to the point that the Republic of Congo has banned the use of this term by law and suggested “autochtone.” In our experience, the Mbendjele of Congo calls themselves and their neighbors the “BaYaka.” To them, the BaYaka include the Mbendjele from the Republic of Congo, the Baka from Cameroon and the different Aka groups found in Congo and Central African Republic. Different terminologies have been proposed (Bahuchet, 1985; Lewis, 2002). For our present analysis, we use the term “BaYaka” as a generic term for the people living in whole region, and use the term Aka for those living in Central African Republic and the term Mbendjele for those living in northern Congo.
[6] The oblong heart-shaped kernels of the Panda nuts are covered by a hard endocarp germinating along a dehiscent line that is used by the nut crackers. Chimpanzee hit them between two dehiscent lines accessing directly two kernels, while BaYaka aim to extract one kernel after the other; Mbendjele access them from the top of the kernel, while Aka women aim for the basis of the kernel thereby cutting the point where the kernel is attached to the nut at the same time as they open them. This may explain why Aka women are clearly quicker in extracting the kernels of Panda than Mbendjele women (Boesch, personal observation).
{1} These authors contributed equally to this study.
{2} These authors contributed equally to this study.