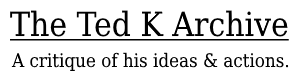Thomas S. Kraft, Vivek V. Venkataraman, Ivan Tacey, Nathaniel J. Dominy & Kirk M. Endicott
Foraging Performance, Prosociality, and Kin Presence Do Not Predict Lifetime Reproductive Success in Batek Hunter-Gatherers
Genealogy and Reproductive Success
Egalitarianism and Social Leveling Mechanisms
Linking Foraging and Reproductive Success
Female Foraging and Reproductive Success
Prosociality: Sharing and Cooperative Foraging
What Can Studies of Reproductive Success Tell Us about Human Behavior and Evolution?
Abstract
Identifying the determinants of reproductive success in small-scale societies is critical for understanding how natural selection has shaped human evolution and behavior. The available evidence suggests that status-accruing behaviors such as hunting and prosociality are pathways to reproductive success, but social egalitarianism may diminish this pathway. Here we introduce a mixed longitudinal/cross-sectional dataset based on 45 years of research with the Batek, a population of egalitarian rain forest hunter-gatherers in Peninsular Malaysia, and use it to test the effects of four predictors of lifetime reproductive success: (i) foraging return rate, (ii) sharing proclivity, (iii) cooperative foraging tendency, and (iv) kin presence. We found that none of these factors can explain variation in lifetime reproduction among males or females. We suggest that social egalitarianism, combined with strikingly low infant and juvenile mortality rates, can mediate the pathway between foraging, status-accruing behavior, and reproductive success. Our approach advocates for greater theoretical and empirical attention to quantitative social network measures, female foraging, and fitness outcomes.
Keywords Hunter-gatherers . Reproductive success . Foraging . Prosociality. Sharing . Cooperation
Foraging ability, prosociality, and coresidence patterns are widely considered to be important drivers of human evolution. For example, causal models of human origins have long emphasized the adaptive significance of hunting, a skill that often entails complex tool use, cooperation, and the sexual division of labor (Dart 1953; Hill 1982;
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12110-018-9334–2) contains supplementary material, which is available to authorized users.
Thomas S. Kraft
tkraft@anth.ucsb.edu
Extended author information available on the last page of the article
Washburn and Lancaster 1968). Smith (2004) reviewed the available evidence linking hunting ability and reproductive success (RS) in five contemporary foraging populations (Ache, Hadza, !Kung, Lamalera, and Meriam) and found consistent evidence of positive correlations between hunting ability and several metrics of reproductive success. Gurven and von Rueden (2006) expanded this analysis and highlighted the overarching role of social status and prestige. The accumulation of status, often separated into dominance (ability to coerce others) and prestige (deference conferred by others), can be achieved through various forms of material, embodied, and relational wealth, factors that positively influence male reproductive success in small-scale societies (Ziker et al. 2016). Recently, von Rueden and Jaeggi (2016) conducted a multilevel phylogenetic meta-analysis focused on the effects of status (including hunting ability) on RS across 46 studies of 33 nonindustrial societies. They found a positive correlation, irrespective of subsistence mode or status measure, suggesting a consistent link between status and differential fitness.
Prosociality and hypercooperation are defining features of humans (Apicella et al. 2012; Chudek and Henrich 2011), and the proximate pathways by which prosociality can influence RS are similar to those investigated for hunting (Gurven and von Rueden 2006): improving status or political influence, obtaining resources or help in times of need, solidifying social support, and attracting mates (Gurven et al. 2014; von Rueden et al. 2008, 2015). Prosociality is difficult to define, however, because it encompasses numerous attributes, such as the proclivity to share resources or provide support to others during conflicts, the donation of public goods, a willingness to give advice or share information, leadership, and cooperative tendencies (von Rueden et al. 2015).
Among Tsimane men, a reputation for meat-sharing is positively related to status, fertility, and the total number of surviving offspring; in fact, the RS of poor hunters who are recognized for sharing meat is comparable to that of skilled but selfish hunters (Gurven and von Rueden 2006). Yet the number of food-sharing partners (von Rueden et al. 2010) and prosocial personality traits (Gurven et al. 2014) did not directly predict RS in the same population. These findings can be reconciled if it is sharing depth, rather than breadth, that defines an association between sharing and RS. Von Rueden et al. (2010) also found that men with greater prestige (characterized by high communitywide influence) have higher fertility, children with lower mortality, and more extrapair matings. The authors suggest that individuals with high community-wide influence are those “whose skills and prosociality provide direct benefits to long-term cooperative partners,” in accordance with previous work that found an indirect relationship between prosocial personality and community-wide influence (von Rueden et al. 2008). Taken together, this work suggests that prosociality (as measured holistically using survey questions of diverse attributes, such as the tendency to keep promises, trustworthiness, giving good advice, willingness to lend money, meat-sharing proclivity, sense of humor, and visitation rates) can lead to increased social support, which is linked to higher status and reproductive success.
Finally, although hunter-gatherer bands contain a large number of unrelated individuals (Hill et al. 2011), interactions with kin occur daily, and kin presence can affect important outcomes, such as child survival (Sear and Mace 2008) and age-specific fertility (Hill and Hurtado 1996). Further, generosity in economic games (Apicella et al. 2012), food sharing (Gurven et al. 2000, 2001; Nolin 2010), and partner choice (Nolin 2011) all tend to be directed preferentially toward kin. As such, kin presence is an important potential predictor of reproductive success.
The goal of the present paper is to evaluate the adaptive significance of foraging return rate, sharing proclivity, cooperative foraging network centrality, and kin presence in a population of egalitarian hunter-gatherers, the Batek of Peninsular Malaysia, using direct measurements of lifetime reproductive success (LRS). We conducted analyses for both males and females. To our knowledge, this is the first study to examine the relationship between foraging return rate and RS in female foragers.
Here we employ the “backward method” of hypothesis development and testing (sensu Sherman and Reeve 1997) by measuring how current behaviors affect RS. This contrasts (but is not mutually exclusive) with the “forward method,” in which a researcher infers the features of a relevant evolutionary environment and the fitness of different phenotypic variants in that environment before asking whether observed phenotypes are those hypothesized to have the highest fitness (Sherman and Reeve 1997). The concept of RS has been controversial in the scientific literature, often because of the different definitions that have been used (Crognier 2003). Here we define RS in biological terms as the number of offspring produced over the life course (fertility) and the number of offspring surviving past the age of five (number of surviving offspring). Some studies employ age-corrected metrics, but lifetime reproductive success is a superior measure of fitness (Clutton-Brock 1988; Sherman and Reeve 1997).
Methods
Study Population and Location
The Batek are one of several indigenous groups living in Peninsular Malaysia, collectively termed Orang Asli (“original people” in Malay). The Batek are also categorized as “Semang,” a Malay exonym and subset of Orang Asli with differentiating phenotypic traits such as short stature, curly hair, and dark skin (Endicott 2013). The Batek are also linguistically distinct, speaking a language in the Aslian branch of the Austroasiatic (Mon-Khmer) language family (Benjamin 1976). The evidence suggests that Semang peoples are united by a deep ancestry, dating to the initial dispersal of modern humans into Peninsular Malaysia >50 kya (Aghakhanian et al. 2015). The Semang practice a foraging lifeway that has included trade with agricultural peoples for thousands of years (Dunn 1975).
The present study is based mainly on fieldwork that was performed in 1975–1976 by Kirk and Karen Endicott with a focal group of Batek De, a dialectic subgroup that lives in the north-central region of Peninsular Malaysia, mainly in the states of Kelantan and Pahang. During 1975–1976, the focal group lived along the upper reaches of the Lebir River. The area was primarily covered with tropical lowland dipterocarp rain forest interspersed with a series of rivers and their tributaries. Rainfall averages ~2270 mm/yr and is seasonal (rainy season: November-January/February; dry season: February-March/April), and temperatures generally range between ~20 and 35° C (Suratman et al. 2012). Food availability in Malaysian rain forests is likewise seasonal, with scarcity during the rainy season and great abundance during the fruit and honey seasons (June-August).
In the 1970s the Batek economy revolved around a traditional nomadic huntergatherer lifestyle that included hunting, fishing, and gathering (Endicott and Endicott 2008; Lye 2004). Common sources of meat included small game such as monkeys, gibbons, squirrels, civets, birds, bamboo rats, and porcupines. Honey from the giant Asian honeybee (Apis dorsata) and fruit were seasonal and appeared between April and August. The most common tubers were wild yams of the genus Dioscorea (~10 species), which provided a stable carbohydrate source throughout the year. In addition, the Batek collected and traded non-timber forest products (primarily rattan, climbing palms in the tribe Calameae, which are valued for use in making furniture and fish traps) with Malay traders. On rare instances during the study period, the Batek also engaged in wage labor or small-scale agriculture (Endicott 1984). These practices have become increasingly common, particularly among Batek individuals who no longer have frequent contact with the rain forest. The study area was composed of primary rain forest in 1975–1976, but since that time, extreme deforestation in this part of Malaysia (Hansen et al. 2013; Sodhi et al. 2004) has forced many Batek to settle outside the rain forest, with the remainder of the population moving inside the protected areas of Taman Negara National Park. These changes have resulted in an economy that includes a greater degree of trade than that observed in the 1970s (Endicott and Endicott 2008).
In 1975–1976, Batek groups occupied temporary foraging camps as they moved nomadically through the rain forest, staying an average of 8.2 days (SD = 6.6) in each camp (Endicott and Endicott 2008; Lye 1997, 2004). Average camp size for the Batek living on the Lebir River in 1975–1976 was 34.2 individuals (11.3 men, 8.6 women, and 14.4 children under the age of 14) (Endicott 1984). Although the focal group of Batek in this study consisted of a core group of coresident individuals, group membership in the Batek was fluid, and people cycled in and out of camps to seek foraging opportunities or to visit relatives and friends. As with most small-scale societies, the Batek are a natural fertility population (Campbell and Wood 1988; Kelly 2013).
The Batek are perhaps best known for their egalitarian norms and gender relations (Endicott and Endicott 2008). Both women and men play a prominent role in the economy, individual autonomy is a highly valued trait, and sharing is widespread. For a more complete review and description of the Batek economy, culture, and lifestyle, see Endicott (1984), Endicott and Endicott (2008), and Lye (2004).
Foraging Data
Foraging data were collected between September 1975 and June 1976 (n = 93 days) by the Endicotts (Endicott and Endicott 2008). During this period, the Batek were living nomadically in the rain forest and engaging in hunting and gathering for subsistence and the collection of rattan for trade. The Batek consumed ~60% of total calories from wild foods and ~40% of total calories from agricultural products (mostly rice), obtained mainly by collecting and trading rattan (Endicott and Endicott 2008).
The Endicotts recorded all food acquisition events from a central location on a daily basis. They observed and interviewed subjects before and after foraging activities to assess foraging goals/outcomes and, when possible, they timed individual foraging bouts (~50% of instances). Spring scales were used to weigh all foods acquired upon return to camp. Observations indicated that the Batek consumed only negligible amounts of food (mostly fruit and pith) during foraging trips.
The caloric values of foods were estimated from raw return weights using standard conversions and measured proportions of waste product for individual items (Venkataraman et al. 2017). Rattan was an important component of the Batek economy and subsistence strategy; it was either bartered for food (mainly rice) or exchanged for cash, which was used immediately to purchase food (Endicott and Endicott 2008). In order to establish comparability with wild food items, we converted rattan to its market value in cash and then to kilocalorie units of rice.
All analyses of foraging data are presented here as return rates, measured in kilocalories/bout. A “bout” here refers to a single foray from camp; thus it was possible for multiple bouts to occur on the same day for an individual forager (although this was exceedingly rare, and a bout is essentially synonymous with a foraging day). There were very few instances in which multiple types of resources (e.g., tubers and meat) were acquired on the same foray, and such cases were considered as separate bouts for both activities. Return rates represent acquisition only and do not include processing costs. Although data on time spent foraging (in hours) was available in ~50% of cases, the use of this metric would have required a substantial loss of data, and per hour foraging return rates correlate tightly with per bout return rates for all resources (e.g., r = 0.81, p < 0.001 for per bout vs. per hour hunting/fishing return rates for males and females). We also conducted analyses using timed return rates, and no results changed substantially. We calculated foraging return rate for individual resource types as well as composite categories. The category “wild food” includes all resources harvested except for rattan; “wild food + rattan” includes all resources including rattan; “gathering” includes collection of tubers, fruit, pith, and honey; and “hunting/ fishing” includes all meat and fish products. Honey collectors were distinguished from non-honey collectors based on whether an individual harvested honey at least one time.
When we examined seasonality in return rates in order to assess whether individuals would be strongly affected by date of presence in our study, we found that overall rate of harvest or failure rate did not differ substantially across time (Fig. S1 in the ESM).
Sharing
The Endicotts systematically recorded all sharing interactions among the Batek on a daily basis through direct observation and interviews. Individuals were asked about all foods consumed and the origin of those foods. There were undoubtedly instances that were not reported (and thus our records are likely to be underestimates), but the systematic nature of data collection makes bias unlikely. Amounts of food transferred could not be adequately assessed, and therefore sharing interactions were coded as a binomial variable (yes/no) by food type. In some instances, a cooperative foraging party was responsible for obtaining food collectively that was then shared, and it was impossible to discern which individuals instigated the food transfer. In those cases sharing interactions were scored as occurring between all pairwise combinations of individuals in the foraging and receiving parties.
We assembled daily sharing networks in R using the igraph and statnet packages (Csardi and Nepusz 2006; Handcock et al. 2008). Nodes in sharing networks represented individuals and directed ties represented the transfer of food from ego to alter on a given day. We generated separate sharing networks for all resource types and categories.
For daily sharing networks, we calculated three metrics for each node (person) present on a given day indicating the propensity of that node to engage in sharing: in-degree, out-degree, and total-degree. In-degree represents the number of individuals sharing food with ego, out-degree represents the number of individuals with whom ego shares food, and total-degree represents the sum of in-degree and out-degree. We calculated in-, out-, and total-degree for each day that a given forager was present and averaged each metric over all camp-days. Thus, degree metrics were not penalized because of absence from camp.
To further examine sharing centrality, we generated a weighted cumulative sharing network based on all days of data collection. In order to evaluate total engagement in sharing (giving and receiving), directed ties in this network were converted to undirected ties by summing ties directed to and from each node. In this network, the strength of an undirected tie corresponds to the number of sharing interactions that occurred between an ego and alter pair over all days of study. We then calculated eigenvector centrality, a metric that takes into account the centrality of nodes to which a central node is connected. Eigenvector centrality provides a strong alternative metric to degree because it takes overall network topology into account. We also generated a directed (asymmetrical) cumulative sharing network and calculated in- and out-eigenvector centrality and Bonacich centrality (Bonacich and Lloyd 2001), but analyses using these variables are not presented because results were not different from our original network analysis.
Cooperative Foraging
Cooperative foraging was recorded on a daily basis by observing which individuals left camp together to go foraging and through post-hoc interviews with foragers about cooperation during resource acquisition. Only cooperation between adults was considered.
We generated daily cooperative foraging networks following the same methods detailed above for sharing networks. In cooperative foraging networks, nodes represented individuals and ties represented a cooperative foraging event for a given resource on a given day. Unlike sharing networks, which are asymmetrical given the directional nature of food transfers, cooperative foraging networks are undirected. We calculated the degree (number of individuals with whom ego cooperates) of each individual for daily cooperative foraging networks and averaged across all days ego was in camp. This procedure was repeated for all resource types and combinations. We also generated cumulative cooperative foraging networks and calculated the eigenvector centrality of nodes.
Kin Presence
We used two metrics of kin presence in this study: average in-camp relatedness and average number of in-camp primary kin. We first generated a complete matrix of coefficients of relatedness using our extensive genealogy (described below). Average in-camp relatedness was calculated by computing the mean relatedness between an individual and all other coresidents on each day, and then averaging across all campdays. We calculated the average number of in-camp primary kin by measuring the number of coresident primary kin (r = 0.5 or half-siblings) on each day and averaging across all camp-days.
Genealogy and Reproductive Success
A long-term collaborative effort has culminated in a Batek genealogy spanning six generations and totaling >1000 individuals. The Endicotts collected genealogical information for the Batek of Kelantan (including all the focal individuals from the main study period) in 1971–1972, 1973, 1975–1976, 1981, 1990, and 2004 (Endicott and Endicott 2008). In addition, IT collected data from 2007 to 2014, and VVV and TSK conducted field research with the Batek of the Lebir region between 2013 and 2016.
Measuring reproductive success is difficult in human systems owing to long lifespans, and studies of lifetime reproductive success in foraging peoples are few. The longitudinal nature of our genealogical data set makes it possible to calculate lifetime reproductive success for all the foragers in this study. Because these individuals were at least 14 years of age in 1975, and our last year of data collection was 2016, we have reproductive history information up to a minimum of age 55. This value is similar to the age at which reproduction is generally completed (Smith 2004). Most individuals exceeded 20 years of age at the time of the foraging study, and we are therefore confident that we observed the entire reproductive life course of each focal individual.
We use two metrics to represent reproductive success. First, we calculated fertility as the total number of offspring an individual produced. Second, we calculated the number of surviving offspring, defined as the number of offspring that survived past age five (Smith et al. 2010). Although it is impossible to be certain about paternity, the Batek are generally monogamous and informants explicitly noted when children were the product of previous partnerships.
Statistical Analysis
We restricted all analyses to adults (>14 years of age) and individuals who were present on more than 5 study days. Sample sizes were 25 and 19 for men and women, respectively, and subjects were observed for an average of 39.7 days (SD = 19, range = 5–84). To ensure that the number of sample days was not driving our results, we re-ran our analyses with a minimum cutoff of 10 study days and did not observe changes to any of our results. To further investigate sample size effects, we also ran power analyses based on effect sizes previously reported in the literature (see “Discussion”).
Although we did not need to control for age effects on reproductive success because of the use of LRS as a response variable, it was necessary to correct for age-related differences for all independent variables. For example, plots of return rate versus age consistently revealed negative quadratic relationships (middle-aged foragers exhibited higher average return rates than young or old foragers), but the shape of age relationships varied by case. We thus controlled for the effects of age by fitting a locally weighted (loess) regression of each independent variable as a function of age (at the time of the 1975–1976 study period) and extracting standardized residuals from this model. Standardized residuals were then used as age-corrected versions of independent variables.
Next, we fit a series of Poisson generalized linear models (GLMs) with standardized age-corrected independent variables as predictors of reproductive success (fertility or number of surviving offspring). We fit separate models for each independent variable given our modest sample sizes. Male and female data were analyzed separately. All models also included an additional term, “age at last observation,” which represented age at death or the last age at which we were able to collect genealogical information. Including the “age at last observation” controlled for the effects of death or disappearance prior to reproductive senescence.
After the original GLMs were fit, we tested for the presence of overdispersion using the dispersiontest function in the AER package, which utilizes a regression-based approach (Cameron and Trivedi 1990). If we detected significant (p<0.05 under the null hypothesis that variance is equal to the mean) overdispersion, we employed negative binomial regressions (NB GLMs). A negative binomial GLM uses maximum likelihood to estimate a scaling parameter, w, which is used to account for the fact that the expected variance increases faster than the mean. Thus, we report the type of model used for final analyses in addition to model coefficients, standard error, and p values.
Data and Code Availability
All analyses were performed in R version 3.1.3 (R Core Team 2015). Data and code associated with this project are available at https://github.com/ThomasKraft/Batek_RS.
Results
Foraging Return Rate
Foraging return rate, fertility, and the number of surviving offspring varied greatly across individuals. Average wild food return rate for men varied between 8589 and 15940 kcal/bout (mean = 7085 kcal/bout) and for women between 1343 and 8527 kcal/bout (mean = 3237 kcal/bout). Average fertility for men and women was 7.1 (range = 0–17) and 7.7 (range = 0–17) offspring, respectively. Average number of surviving offspring for men and women was 6.0 (range = 0–12) and 6.2 (range = 0–12), respectively.
For all resource types and categories, age-corrected foraging return rate was not correlated (p>0.05) with fertility or the number of surviving offspring for both sexes, or coefficients indicated a negative relationship (Fig. 1; Tables 1, S1). We consider the few significant finding s in the negative direction to be spurious correlations resulting from the large number of statistical tests. Honey collectors did not have greater fertility (t33.4 = -1.27, p = 0.21) or number of surviving o ffspring (t30.3 = -1.09, p = 0.29) than non-honey collectors.
In order to determine whether seasonal effects or heterogeneity across time affected the relationship between foraging and reproductive success, we also calculated daily residuals in foraging rates across individuals and tested whether there was a relationship with reproductive outcomes. There was no evidence that foragers who did better than their peers on a daily basis achieved higher reproductive success. Results obtained using this approach therefore did not differ from those presented above, and the high similarity in outcomes increases our confidence that foraging data were not biased by the time it was collected during the study.
Sharing
Average daily in, out, and total sharing degree for men were 0.83 (range = 0.10–1.38), 0.87 (range = 0.05–2.27), and 1.7 (range = 0.16–3.24), respectively. For women, these values were 0.89 (range = 0.35–1.42), 0.69 (range = 0.20–1.98), and 1.58 (range = 0.80–3.18), respectively.
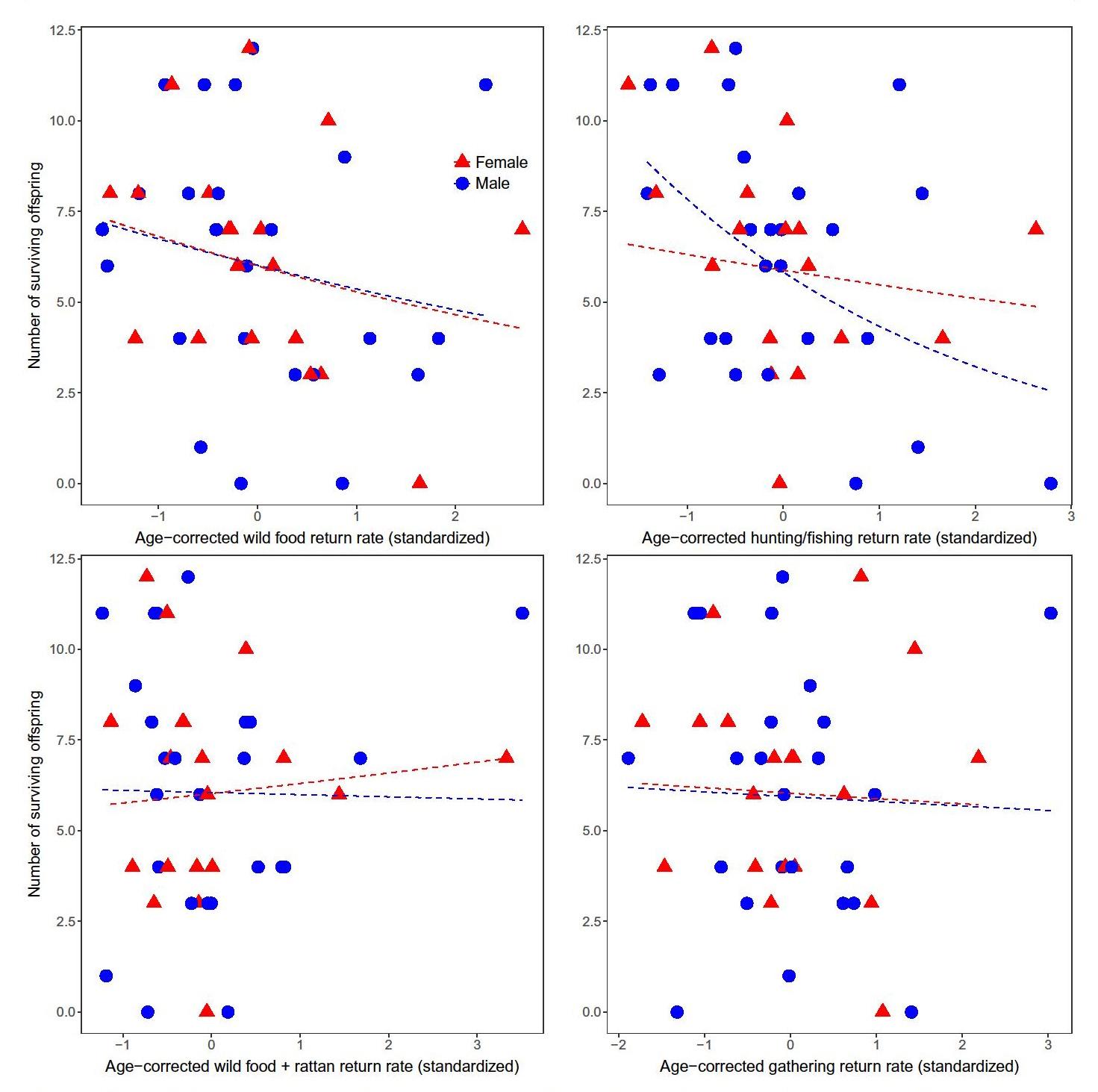
For all resource types and categories, mean in-degree, out-degree, and total-degree calculated across daily sharing networks were uncorrelated (p >0.05) with fertility or
Table 1 Statistical models of the association between age-corrected foraging return rates and reproductive success (measured as either lifetime fertility or the lifetime number of offspring surviving past age 5) for specific resource categories. Slopes, standard errors, and p values are from either generalized linear models (GLM) or negative binomial GLMs (NB GLM) controlling for the age of last observation the number of surviving offspring (Fig. 2; Tables 2, S1). Likewise, eigenvector centrality for the cumulative sharing network was uncorrelated with fertility or the number of surviving offspring.
| Fertility | N of Surviving Offspring | |||||||||||||||
| Male | Female | Male | Female | |||||||||||||
| P (SE) | P | n | model | P (SE) | P | n | model | P (SE) | P | n | model | P (SE) | P | n | model | |
| Blowpipe hunting | -0.28 (0.14) | 0.0505 | 21 | NB GLM | 0.12 (0.25) | 0.639 | 4 | GLM | -0.2 (0.13) | 0.144 | 21 | NB GLM | 0.079 (0.26) | 0.758 | 4 | GLM |
| Other hunting | -0.27 (0.16) | 0.0928 | 19 | NB GLM | 0.13 (0.16) | 0.398 | 10 | GLM | -0.17 (0.15) | 0.251 | 19 | NB GLM | 0.14 (0.17) | 0.388 | 10 | GLM |
| Tubers | 0.12 (0.13) | 0.374 | 22 | NB GLM | -0.22 (0.096) | 0.0217 | 19 | GLM | 0.067 (0.13) | 0.599 | 22 | NB GLM | -0.13 (0.1) | 0.194 | 19 | GLM |
| Fishing | 0.18 (0.11) | 0.115 | 25 | NB GLM | -0.062 (0.086) | 0.467 | 19 | GLM | 0.11 (0.11) | 0.302 | 25 | NB GLM | 0.0021 (0.092) | 0.982 | 19 | GLM |
| Rattan | -0.053 (0.13) | 0.678 | 25 | NB GLM | 0.37 (0.27) | 0.17 | 7 | GLM | -0.0023 (0.12) | 0.984 | 25 | NB GLM | 0.11 (0.29) | 0.711 | 7 | GLM |
Cooperative Foraging
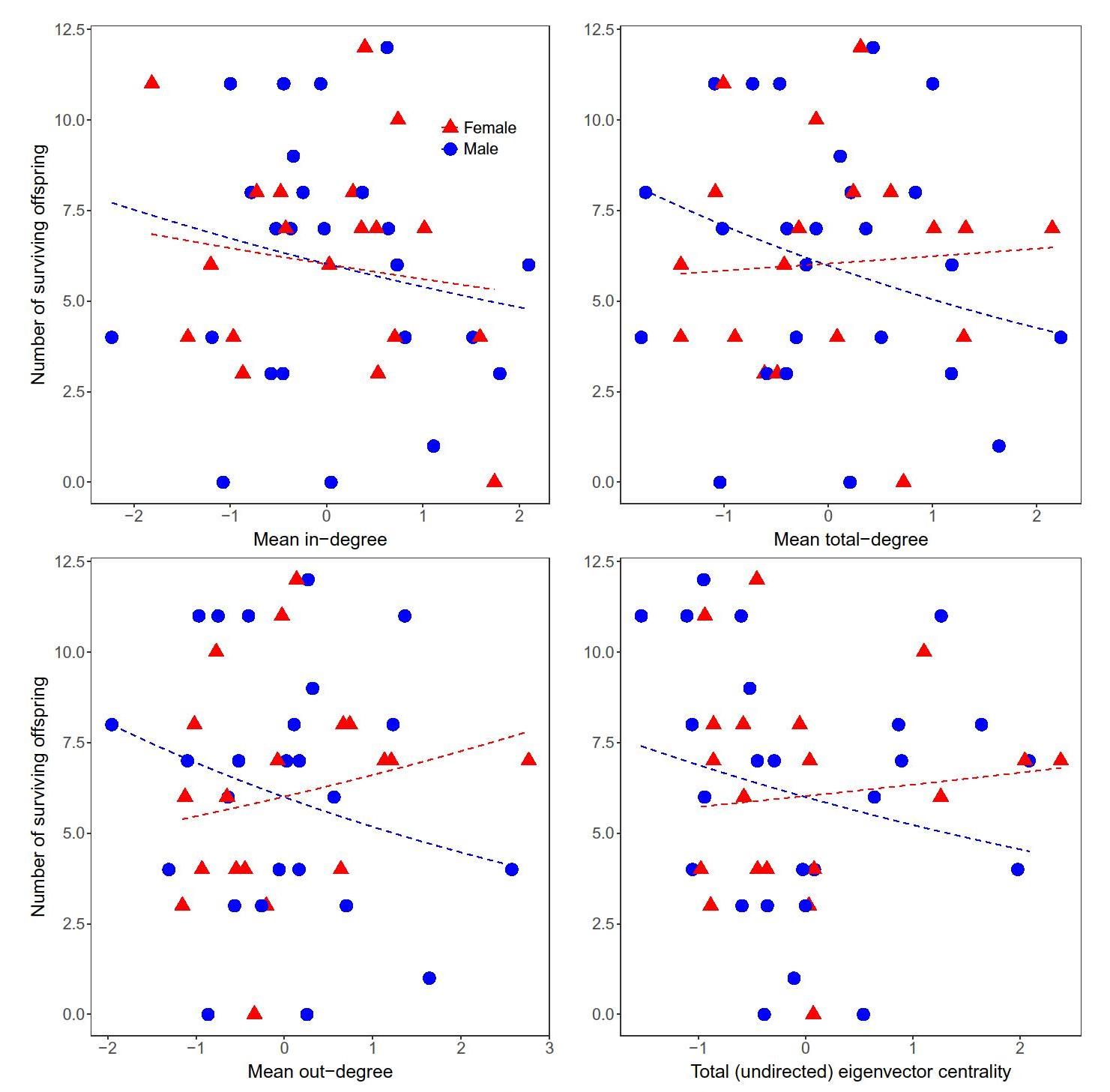
For cooperative foraging networks of all resource types, average daily degree was 0.46 (range = 0.08–1.05) and 0.47 (range = 0.03–0.77) for men and women, respectively. Average eigenvector centrality for the cumulative cooperative foraging network for all resources combined was 0.26 (range = 0.003–1.0) and 0.17 (range = 0.001–0.573) for men and women, respectively.
Both mean degree (from daily networks) and eigenvector centrality (from the cumulative network) were uncorrelated (p > 0.05) with fertility or the number of surviving offspring (Fig. 3, Table S1). Mean degree and eigenvector centrality for specific resource networks were also uncorrelated with fertility and the number of surviving offspring except for two marginally significant cases (Table 3). We consider these results, which are infrequent and inconsistent in direction, to be spurious correlations resulting from the large number of statistical tests.
Table 2 Statistical models of the association between age-corrected sharing network metrics and reproductive success (measured as either lifetime fertility or the lifetime number of offspring surviving past age 5) for specific resource categories. Slopes, standard errors, and p values are from GLMs or NB GLMs controlling for the age of last observation
| Fertility | N of Surviving Offspring | |||||||||||||||||
| Male | Female | Male | Female | |||||||||||||||
| Resource |
Dependent Variable |
3 (SE) | p | n | model | P (SE) | p | n | model | P (SE) | p | n | model | P (SE) | p | n | Model | |
| Primate meat | In-degree | 0.052(0.13) | 0.68 | 25 | NB GLM | 0.047 (0.085) | 0.578 | 19 | GLM | -0.0013 (0.12) | 0.991 | 25 | NBGLM | 0.072 (0.094) | 0.445 | 19 | GLM | |
| Out-degree | -0.12 (0.13) | 0.358 | 25 | NB GLM | -0.011 (0.092) | 0.906 | 19 | GLM | -0.049 (0.12) | 0.678 | 25 | NBGLM | 0.025 (0.097) | 0.795 | 19 | GLM | ||
| Total-degree | -0.11 (0.13) | 0.397 | 25 | NBGLM | 0.046 (0.085) | 0.589 | 19 | GLM | -0.052 (0.12) | 0.665 | 25 | NBGLM | 0.073 (0.094) | 0.44 | 19 | GLM | ||
| Other meat | In-degree | -0.11 (0.14) | 0.425 | 25 | NBGLM | 0.0089 (0.085) | 0.917 | 19 | GLM | -0.073 (0.12) | 0.557 | 25 | NB GLM | 0.069 (0.092) | 0.457 | 19 | GLM | |
| Out-degree | -0.073 (0.13) | 0.572 | 25 | NBGLM | 0.035 (0.082) | 0.664 | 19 | GLM | -0.00098 (0.12) | 0.993 | 25 | NBGLM | 0.024 (0.091) | 0.789 | 19 | GLM | ||
| Total-degree | -0.083 (0.13) | 0.535 | 25 | NB GLM | 0.033 (0.089) | 0.712 | 18 | GLM | -0.0041 (0.12) | 0.973 | 25 | NB GLM | 0.082 (0.098) | 0.402 | 18 | GLM | ||
| Gathered food | In-degree | -0.06 (0.14) | 0.662 | 25 | NBGLM | -0.06 (0.092) | 0.513 | 19 | GLM | -0.048 (0.13) | 0.701 | 25 | NBGLM | -0.033 (0.1) | 0.744 | 19 | GLM | |
| Out-degree | -0.097 (0.13) | 0.448 | 25 | NBGLM | 0.11 (0.085) | 0.177 | 19 | GLM | -0.089 (0.12) | 0.448 | 25 | NBGLM | 0.13 (0.093) | 0.162 | 19 | GLM | ||
| Total-degree | -0.093 (0.13) | 0.481 | 25 | NBGLM | 0.048 (0.087) | 0.581 | 19 | GLM | -0.082 (0.12) | 0.495 | 25 | NBGLM | 0.073 (0.096) | 0.442 | 19 | GLM | ||
| Fish | In-degree | 0.035 (0.13) | 0.781 | 25 | NB GLM | 0.028 (0.086) | 0.74 | 19 | GLM | 0.037 (0.12) | 0.754 | 25 | NB GLM | -0.0039 (0.095) | 0.968 | 19 | GLM | |
| Out-degree | 0.17(0.11) | 0.121 | 25 | NB GLM | -0.076 (0.084) | 0.365 | 19 | GLM | 0.11 (0.1) | 0.3 | 25 | NB GLM | -0.067 (0.092) | 0.471 | 19 | GLM | ||
| Total-degree | 0.15 (0.11) | 0.166 | 25 | NB GLM | -0.024 (0.083) | 0.772 | 19 | GLM | 0.1 (0.1) | 0.325 | 25 | NBGLM | -0.042 (0.091) | 0.647 | 19 | GLM | ||
| Agricul tural/S tore-bou ght | In-degree | -0.056 (0.13) | 0.662 | 25 | NB GLM | 0.046 (0.084) | 0.582 | 19 | GLM | -0.028 (0.12) | 0.813 | 25 | NBGLM | -0.0018 (0.093) | 0.985 | 19 | GLM | |
| Out-degree | -0.028 (0.13) | 0.828 | 25 | NB GLM | 0.036 (0.083) | 0.66 | 19 | GLM | -0.093 (0.12) | 0.44 | 25 | NBGLM | 0.048 (0.09) | 0.592 | 19 | GLM | ||
| Total-degree | -0.054 (0.13) | 0.679 | 25 | NBGLM | 0.05 (0.083) | 0.548 | 19 | GLM | -0.1 (0.12) | 0.38 | 25 | NBGLM | 0.038 (0.092) | 0.683 | 19 | GLM |
Kin Presence
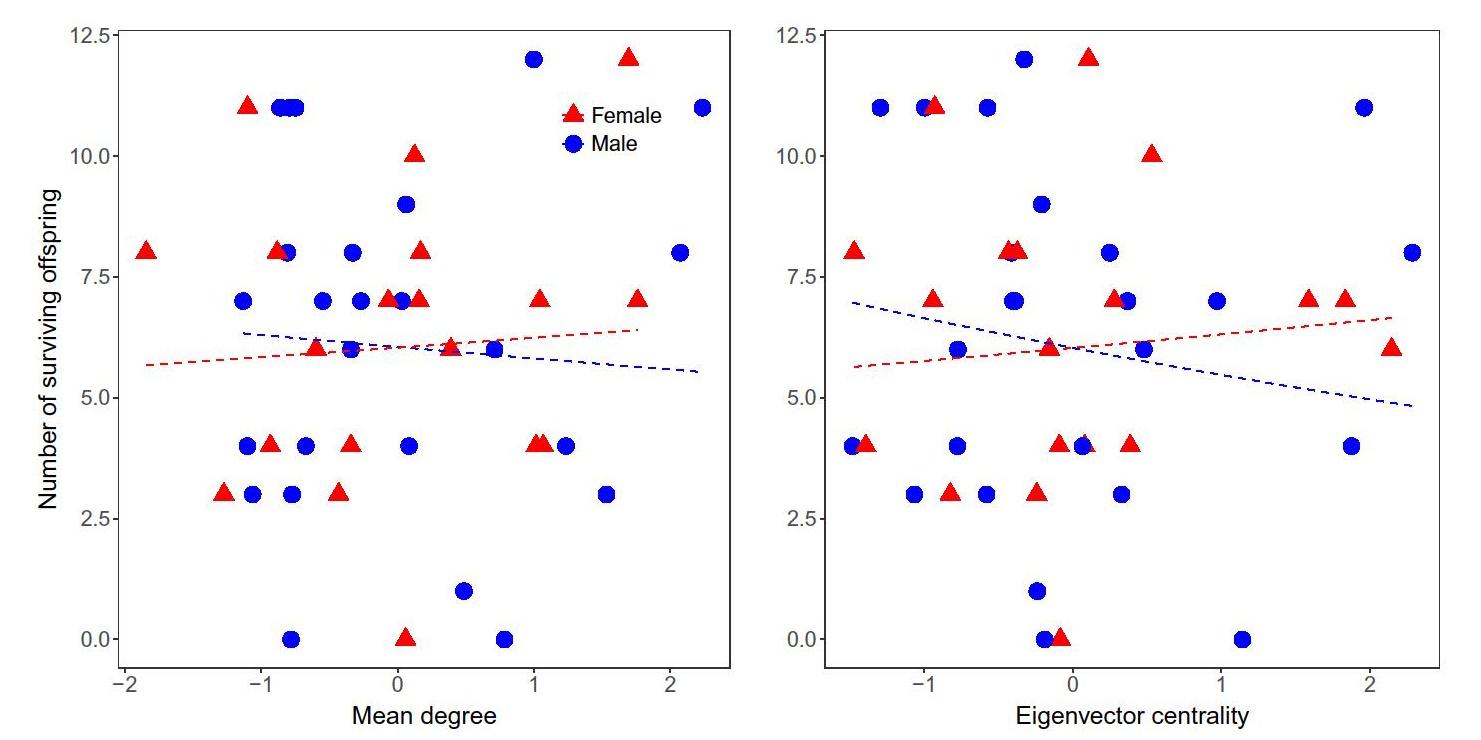
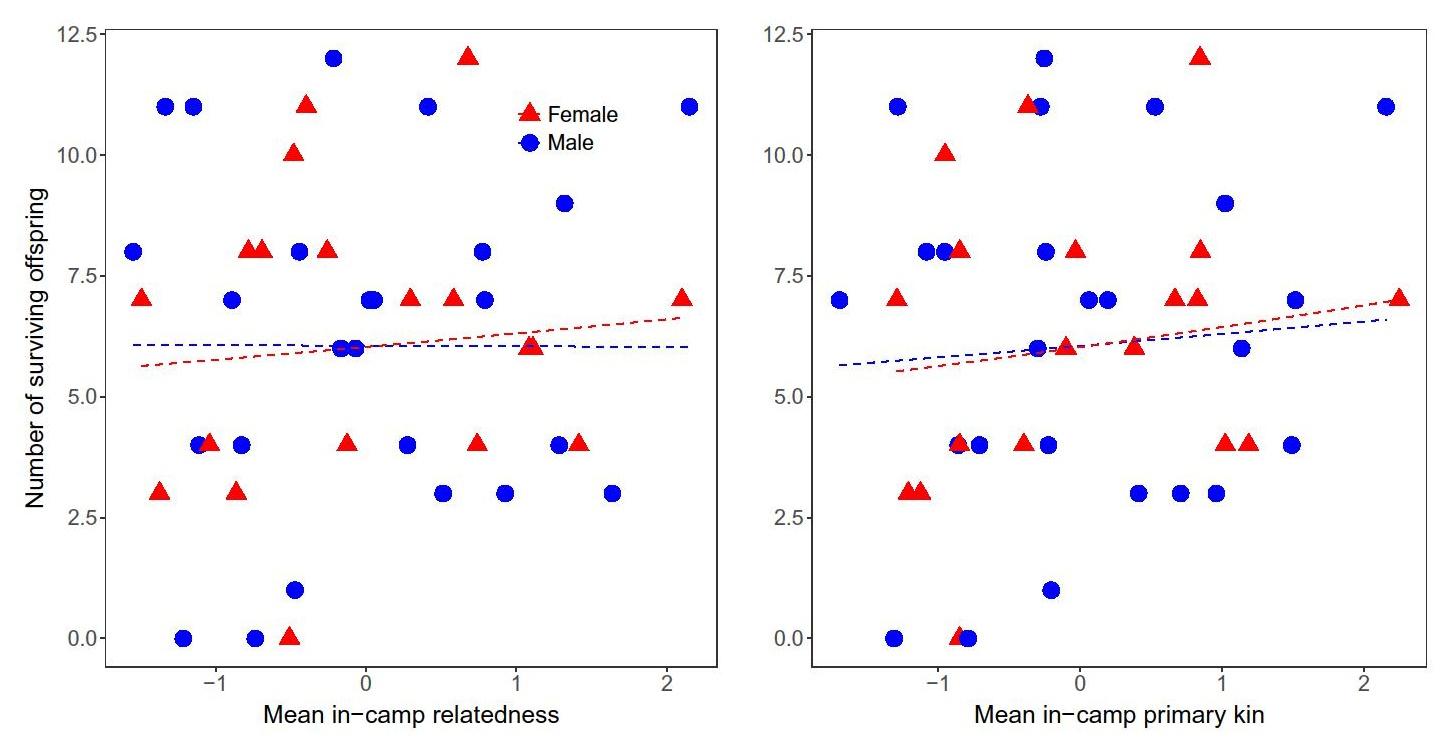
Kin presence was variable across individuals: mean relatedness to other camp members was 0.075 (range = 0.0–0.16), and mean number of coresident primary kin was 2.0 (range = 0.0–4.6). Generalized linear models showed that average number of coresident primary kin was unrelated to fertility or number of surviving offspring for both males and females (Fig. 4, Table S1). Similarly, average in-camp relatedness was not a significant predictor of fertility or number of surviving offspring (Fig. 4, Table S1).
Discussion
We analyzed foraging return rate, sharing proclivity, cooperative foraging network centrality, and kin presence using data collected between 1970 and 2016, and we found that none of these factors were correlated with fertility or the number of surviving offspring in Batek hunter-gatherers. Our results apply to both men and women. These findings are not due to a lack of variation in RS: fertility varied between 0 and 17 (mean = 7.4), and the number of surviving offspring varied between 0 and 12 (mean = 6.2). Despite the absence of clear patterns, these data have important implications for understanding the determinants of RS in small-scale societies.
Table 3 Statistical models of the association between age-corrected cooperative foraging metrics and reproductive success (measured as either lifetime fertility or the lifetime number of offspring surviving past age 5) for specific resource categories. Slopes, standard errors, and p values are from either generalized linear models GLMs or NB GLMs controlling for the age of last observation
| Fertility | N of Surviving Offspring | ||||||||||||||||
| Male | Female | Male | Female | ||||||||||||||
| Resource | Dependent Variable | P (SE) | p | n | model | P (SE) | p | n | model | P (SE) | p | n | model | P (SE) | p | n | Model |
| Hunting | Avg. Degree | -0.085 (0.13) | 0.51 | 25 | NB GLM | -0.035 (0.1) | 0.733 | 19 | GLM | -0.041 (0.12) | 0.733 | 25 | NB GLM | -0.039 (0.11) | 0.734 | 19 | GLM |
| Eigenvector centrality | -0.094 (0.13) | 0.473 | 25 | NB GLM | 0.1 (0.12) | 0.37 | 19 | GLM | -0.025 (0.12) | 0.837 | 25 | NB GLM | 0.078 (0.13) | 0.548 | 19 | GLM | |
| Gathering | Avg. Degree | 0.19(0.11) | 0.0936 | 25 | NB GLM | 0.051 (0.087) | 0.56 | 19 | GLM | 0.18 (0.07) | 0.009 | 25 | GLM | 0.054 (0.096) | 0.572 | 19 | GLM |
| Eigenvector centrality | 0.13 (0.12) | 0.275 | 25 | NB GLM | 0.02 (0.082) | 0.806 | 19 | GLM | 0.14 (0.11) | 0.21 | 25 | NB GLM | 0.053 (0.09) | 0.551 | 19 | GLM | |
| Fishing | Avg. Degree | 0.2 (0.12) | 0.0769 | 25 | NB GLM | -0.099 (0.088) | 0.26 | 19 | GLM | 0.16 (0.11) | 0.141 | 25 | NB GLM | -0.078 (0.096) | 0.415 | 19 | GLM |
| Eigenvector centrality | -0.16(0.12) | 0.161 | 25 | NB GLM | 0.046 (0.084) | 0.579 | 19 | GLM | -0.15 (0.075) | 0.0396 | 25 | GLM | 0.043 (0.093) | 0.646 | 19 | GLM | |
| Honey | Avg. Degree | -0.18(0.13) | 0.165 | 25 | NB GLM | 0.091 (0.11) | 0.423 | 19 | GLM | -0.16 (0.087) | 0.0682 | 25 | GLM | 0.079 (0.13) | 0.527 | 19 | GLM |
| Eigenvector centrality | -0.15 (0.13) | 0.231 | 25 | NB GLM | 0.08 (0.11) | 0.46 | 19 | GLM | -0.1 (0.12) | 0.398 | 25 | NB GLM | 0.06 (0.12) | 0.618 | 19 | GLM | |
| Rattan | Avg. Degree | -0.17(0.12) | 0.151 | 25 | NB GLM | -0.035 (0.089) | 0.695 | 19 | GLM | -0.11 (0.11) | 0.303 | 25 | NB GLM | 0.0096 (0.096) | 0.92 | 19 | GLM |
| Eigenvector centrality | -0.23 (0.13) | 0.0676 | 25 | NB GLM | -0.027 (0.089) | 0.759 | 19 | GLM | -0.15 (0.12) | 0.201 | 25 | NB GLM | 0.013 (0.096) | 0.897 | 19 | GLM |
Egalitarianism and Social Leveling Mechanisms
Evolutionary theory focuses on the process by which organisms acquire nutrients and convert those nutrients into genetic copies (offspring). Organisms that are superior at acquiring and assimilating nutrients are therefore predicted to achieve higher reproductive success by producing more (surviving) offspring.
If Batek hunter-gatherers experience high variability in both foraging return rates and offspring production, why then do better foragers not experience greater reproductive success? One explanation could be that the reproductive success of human foragers is dependent on other social factors that influence the availability of resources. Yet this study was designed to test the importance of several such social factors that have a theoretical link to reproductive success, including sharing proclivity, cooperative foraging tendency, and kin presence, and we found no evidence of positive relationships.
An alternative explanation is that the Batek culture undermines the kinds of social distinctions that allegedly led to higher reproductive success in some other hunting and gathering societies (K. L. Endicott and K. M. Endicott 2014; Endicott 1979, 2011; K. M. and K. L. Endicott 2008; Lye 2004). Widespread food-sharing is expected to diminish advantages that the best food-getters might have in nourishing their offspring and other relatives. Batek also do not have any political positions giving some people prestige or power over others. Even government-appointed “headmen” do not have any authority within Batek society. Batek are taught from an early age to respect the personal autonomy of all other Batek, young and old, male and female. Batek condemn attempts to coerce or pressure others to do anything they do not want to do. They believe such acts would put the victim in danger of contracting a serious disease or having an accident. Males and females have equal autonomy, including equal say in decisions about choice of spouse and reproductive matters. Most daily decisions about foraging activities, movements, and so on, are made by spouses together, and both parents cooperate in raising their children with help from other camp members. In the 1970s there were no substantial enduring differences in material wealth among individuals or families. People owned only a few personal possessions, food was immediately shared rather than stored, and most items of equipment obtained from traders did not last long in the tropical forest conditions. Although Batek took quiet satisfaction in their skills and accomplishments, there was a strong social convention against overt bragging or showing off. Modesty was a valued trait. Hunters with game would usually enter camp quietly and then hand the carcass to someone else to butcher and distribute. Gatherers with loads of tubers would distribute their surplus to other families without fanfare. Although Batek strongly guarded their personal autonomy, they also felt an obligation to cooperate with other camp and group members. Competition was suppressed, even in games adopted from outsiders, such as cards.
Comparative data are necessary to test the hypothesis that social leveling mechanisms in egalitarian societies could suppress the relationship between status and reproductive outcomes. The only such attempt is a recent meta-analysis which found that there was a positive relationship between status and reproductive success across 33 nonindustrial societies (von Rueden and Jaeggi 2016). In one of their main findings, the authors purported to test a hypothesis about social organization (the “egalitarianism hypothesis”) by asking whether foragers evince a similar correlation as observed in pastoralist or horticulturalist/agriculturalist societies. Von Rueden and Jaeggi (2016) found no differences between foragers and non-foragers and thereby concluded that status plays a universal role in humans “despite subsistence-associated variation in political egalitarianism.”
Yet many foraging groups, including some in the von Rueden and Jaeggi (2016) sample, are not egalitarian. For example, the Lamalera are “complex marine foragers. They are non-egalitarian, live at a relatively high population density for foragers, are not very mobile, have specialized occupations, corporate descent groups, and food storage” (Alvard 2003:134). Likewise, despite having “no complex or stable political system” and engaging in widespread sharing of marine resources, the Meriam exhibit extreme territoriality, corporate land ownership based on patrilineage, and “during ... public feasting events (and to a lesser extent at other times), men engage in competition involving dancing, sorcery, gardening, hunting, diving, marble shooting, top spinning, and boat racing” (Smith and Bliege Bird 2000:249). In contrast to the Batek case, open engagement in public competitions encourages the development of informal status hierarchies and makes it easier for females to assess the quality of mates or for males to assess the formidability of competitors/allies. Finally, the Dolgan live in large settlements, practice directed food sharing, adhere to organized Christianity, and exhibit wealth accumulation, raising questions about the application of the label “egalitarian” to these traditional reindeer pastoralists (Ziker 2014).
As such, the degree to which concepts of egalitarianism overlap with a nomadic foraging subsistence mode is unclear. Widespread sharing and the inability to accumulate material wealth are commonly used as key indicators of egalitarianism (Woodburn 1982), and Kelly (2013:244) emphasizes the importance of autonomy: “Egalitarian societies are those in which each person has the potential to achieve prestige and where the enforcement of cultural norms prevent a person from using that prestige to gain power over another.” Although such features are undeniably found more commonly among foragers than non-foragers, recent evidence demonstrates that in many foraging societies producers retain at least some control of harvests (Gurven 2004; Ziker et al.
2016), sharing distributions are often preferentially directed toward close kin or reciprocators (Jaeggi and Gurven 2013), and individuals are capable of accumulating and transmitting resources or material/relational wealth (Kelly 2013). These factors provide the necessary ingredients for status inequality, and there is reason to expect that where status can be recognized and quantified by anthropologists, we will observe a general association with reproductive success. In populations where common proxies do not necessarily translate to status, however, the association may be less obvious. Von Rueden and Jaeggi (2016) thus provide convincing evidence that the link between status and reproductive success is invariant across subsistence modes, but not necessarily across social systems.
We propose that a true test of the egalitarianism hypothesis must clearly define the mechanism of egalitarianism and carefully discriminate its presence among societies considered for analysis. To achieve this goal, it will be necessary to make quantitative assessment of (even informal) status differentiation that is comparable across societies. Examples of other factors that could influence status include storytelling knowledge (Smith et al. 2017), religious activity (Singh 2018), or natural charisma (Endicott and Endicott 2008).
Linking Foraging and Reproductive Success
Foraging performance, measured here as foraging return rate (kcal/bout), and its association with RS has been well studied in small-scale societies, including huntergatherers. Most attention has focused on hunting ability, yet we found no evidence of such a relationship in the Batek when considering blowpipe hunting, other hunting, or hunting/fishing together (Fig. 1; Table 1). We consider several reasons for this discrepancy.
First, data limitations may prevent us from being able to discriminate between good and poor foragers. Hill and Kintigh (2009) demonstrated that measurement error in foraging data can cause researchers to underestimate true relationships between foraging and biological outcomes, often because of small sample sizes. Although we cannot definitively rule out the possibility that data limitations constrained our ability to detect relationships between foraging and reproductive success, the amount of data employed here, collected across multiple seasons, strongly suggests that this is not the case. Calculations of 95% confidence intervals around individual return rates demonstrated that we had reasonable power to distinguish good and poor foragers, and that mean rates were not highly biased by number of sampling days (Fig. S2). Furthermore, the resolution of data used here is on par with previous studies that have identified positive relationships. A more detailed explanation can be found in the ESM.
A second possible explanation is that the Batek are fundamentally different than other study populations with respect to factors such as local ecology, social system, and culture. As noted above, social enforcement of egalitarian norms in the Batek may preclude good hunters from accruing benefits that increase RS. Widespread meatsharing is a feature of many hunter-gatherer societies (Kelly 2013), but even so, distribution can be an important signaling mechanism if hunters are recognized providers for the community (Hawkes and Bliege Bird 2002). Yet showing off, bragging, or other displays that broadcast individual foraging success are culturally discouraged among the Batek to an extreme degree (Endicott and Endicott 2008). For instance, when men hunted cooperatively, in some cases they would report that “we got such and such an animal.” They were reluctant to specify who actually shot it until questioned repeatedly. Cultural norms inhibit successful hunters from bragging about their success at the expense of their companions. As such, the Batek themselves could plausibly have trouble distinguishing between individual skill levels, thereby limiting the influence that foraging has on one’s status or prestige.
The Batek are not alone in possessing cultural norms that suppress status or prestige inequality. For example, Ju/’hoansi hunters use self-deprecation or avoid announcing a successful kill to avoid displaying arrogance or authority (Cashdan 1980; Lee 1969, 2003). As such, it is perhaps not surprising that Wiessner (2002) did not find any statistical differences between “good” and “poor” hunters with respect to wife quality, mating success, fertility, or surviving offspring. Kirchengast (2000) also found no relationship between male stature or weight and RS in the !Kung (despite contradictory claims in the text based on flawed statistical analyses and interpretation). Likewise, the marital status of Efe men was not predicted by hunting return rate (Bailey 1991), and fertility was not higher for men occupying the leadership role of kombeti in the Aka (Hewlett 1988). We predict that similar findings may be revealed in other societies for which strong cultural norms diminish the link between foraging and status acquisition, such as the Agta of the Philippines or some foraging groups in central Africa.
Yet previous reports of nonsignificant relationships between hunting and RS differ from the current study in that results often trended in the positive direction. For example, Wiessner (2002) found that good !Kung hunters produced 1.8 more surviving offspring than poor hunters, and Aka kombeti had more children (but not significantly more) than non-kombeti (Hewlett 1988). In contrast to our findings on the Batek, this suggests that previous null results are more likely the result of underpowered statistical analyses.
Third, a strikingly low rate of infant and juvenile mortality may reduce or eliminate some of the key benefits from increased foraging performance. In contrast to other hunter-gatherer populations, whose mortality rates for individuals <15 years of age are on average ~40–50% (Kelly 2013), only 18.4% of children born to foragers in this study died before the age of 15. Most of this difference can be accounted for by a relatively low infant mortality rate (10.4%), which can be 20–30% for some other foragers, such as the Dobe Ju/’hoansi, Hadza, or Agta (Kelly 2013).
Because of low overall infant and juvenile mortality, better Batek foragers may lose the opportunity to distinguish themselves via improved offspring survival resulting from increased provisioning or social benefits, a known pathway by which better hunters achieve higher reproductive success (Smith 2004). Instead, most deaths may arise from exogenous factors unrelated to food acquisition, with fertility largely accounting for differences in reproductive outcomes. A lack of differentiation with regard to offspring survivorship may thus account for discrepancies between the results presented here and those from previous studies.
Fourth, study design and the nature of data collected differ considerably between studies (Table 4). For example, only two of the studies (Ache and Hadza) considered by Smith (2004) used a continuous measure of hunting ability. Other studies divided hunters into good versus bad categories or drew comparisons between individuals who did or did not participate in certain types of hunts (e.g., whale hunts in the Lamalera). In addition, ours is the only study other than the !Kung example (Wiessner 2002) to measure lifetime RS. Other researchers accounted for incomplete reproductive careers by including age as a covariate in statistical models. This approach, while understandable given logistical constraints, may produce biased results given the age-structured fertility schedules of hunter-gatherers (Hill and Hurtado 1996). The ESM contains further discussion of complicating factors in the studies of the Ache, Hadza, and Tsimane that found positive associations between hunting and RS.
Table 4 Summary table of studies examining the relationship between hunting and reproductive success in foragers
| Population | Hunting measure | Continuous?{1} | Reproductive success metric(s) | Sample size{2} | Significant{3} | Study |
| Ache (forest period) | Hunting rank, return rate | Yes | Fertility hazard (age-controlled), offspring mortality hazard (age-controlled), number of mates | 176 | Yes | Hill and Hurtado 1996 |
| Ache (reservation) | Return rate | Yes | Fertility hazard (age-controlled), offspring mortality hazard (age-controlled), number of mates | 157 | No | Hill and Hurtado 1996 |
| Dolgan/Nganasan | Hunting skill rating | Yes | Number of surviving offspring, age of first reproduction | 142 | Yes | Ziker et al. 2016 |
| Hadza | Hunting rank | Yes | Fertility (age-controlled), number of surviving offspring (age-controlled), number of mates, younger mates | 39 | Yes | Hawkes et al. 2001 |
| Hadza | Hunting reputation | Yes | Number of surviving offspring, younger mates | 55 | Yes | Marlowe 2000 |
| Hadza | Hunting reputation | Yes | Fertility (age-controlled) | 54 | Yes | Apicella 2014 |
| Kubo | Return rate /“showoffs” vs. “non-showoffs” | No | Number of surviving offspring | 12 | No | Dwyer and Minnegal 1993 |
| !Kung | Good vs. bad hunters | No | Lifetime fertility, lifetime number of surviving offspring, number of mates, younger mates | 26 | No | Wiessner 2002 |
| Lamalera | Whale hunters vs. non-hunters | No | Fertility (age-controlled), number of surviving offspring (age-controlled), age at first birth, younger mates | 364 | Yes | Alvard and Gillespie 2004 |
| Meriam | Turtle hunters vs. non-hunters | No | Fertility (age-controlled), number of surviving offspring (age-controlled), age at first birth, number of mates, younger mates | 98 | Yes | Bliege Bird et al. 2001; Smith et al. 2003 |
| Tsimane (accultura ted) | Hunting rank | Yes | Fertility (age-controlled), number of surviving offspring (age-controlled), age at first marriage, age at first birth, number of mates | 59 | Yes | Gurven and von Rueden 2006 |
| Tsimane (remote) | Return rate, total returns | Yes | Fertility (age-controlled), number of surviving offspring (age-controlled), age at first marriage, age at first birth, number of mates | 57 | No | Gurven and von Rueden 2006 |
| Batek | Return rate | Yes | Lifetime fertility, lifetime number of surviving offspring | 25 | No | Current study |
There are also important differences between studies that measured foraging directly from observation versus those that measure reputation. Whereas Gurven and von Rueden (2006) found that Tsimane men with higher caloric return rates in remote villages did not have higher RS, men in more acculturated villages with better hunting reputations did have higher RS. Similarly, there was a positive correlation between Ache hunting reputation and RS during the forest period, but no correlation between observed hunting return rate and RS during the reservation period (Hill and Hurtado 1996). As such, reputation may be a better tool for capturing long-term variation in hunting success given the timespan over which hunting behavior is observed (although this approach is not without potential problems, such as short-term “halo” effects reported near successful hunting bouts). Nevertheless, our findings agree with several other studies that employed observed measures of hunting ability.
Fifth, we consider the possibility that the sample size of our historical study limited our ability to identify a relationship between predictor variables and RS. Indeed, two other case studies that failed to identify a link between hunting and RS are notable for their small sample sizes (Dwyer and Minnegal 1993; Kent 1996). To test this possibility, we conducted a power analysis using published estimates of effect sizes in studies of hunting ability and reproductive success in foragers. Von Rueden and Jaeggi (2016) reported an overall effect size of 0.18 for the relationship between status and RS for foragers (95% CI: 0.05–0.32), and this effect was only slightly greater if we narrow this sample to studies where the status measurement was associated with hunting (using the supplemental material from von Rueden and Jaeggi 2016, Zr = 0.20, range = -0.18–0.46). A power analysis of this effect size reveals that a sample size of ~200 would be necessary to obtain a power of 0.8 at a standard alpha level (0.05).[1] Although this finding suggests that our results, and those of other underpowered studies of hunter-gatherers, must be interpreted with some caution, we call attention to two additional points.
First, the highest Zr reported for foragers/hunting in von Rueden and Jaeggi (2016) is 0.46 (from the relationship between offspring survival and RS in Ache men). If we repeat the power analysis procedure using this effect size, then the desired sample size to achieve a power of 0.8 is 34, which is similar to that used here. Our study should therefore have been capable of detecting relatively strong trends, and we are thus confident that any undetected effects would be small. Second, we call attention to the fact that a large proportion of the tests presented in our study are not only statisically insignificant but evince slopes in the opposite direction of those predicted (i.e., negative relationship between hunting return rate and RS; Table 1). Had we observed many positive relationships (in the predicted direction), none of which were “statistically significant,” that would be reason to question seriously the null interpretation of our results. Nonetheless, we acknowledge the difficulties of interpreting null findings or “evidence of absence” when using small sample sizes and reiterate the importance of combining evidence from across sample populations for inference.
A final possibility is that selection for efficient hunting may no longer operate in modern contexts, despite having occurred in past environments (Sherman and Reeve 1997). There is substantial discussion about the ongoing role of natural selection in contemporary human populations and the degree to which changes in subsistence practices, residence patterns, or culture render differential RS irrelevant (Smith et al. 2001; Symons 1989). The nature of Batek foraging might have changed in terms of target resources, technological innovations, or integration with market economies, or RS as measured here may no longer be an appropriate fitness measure (Sherman and Reeve 1997). Although Batek foragers were highly reliant on wild food resources in the 1970s when foraging behavior was studied, LRS for these individuals is an outcome integrated across time periods during which the economy and culture have changed for some individuals (Endicott and Endicott 2008). However, the Batek have not yet undergone a demographic transition as of 2016, and core foraging activities performed in the 1970s still constitute a significant portion of time allocation, despite overall changes in the degree of market integration and acculturation occurring in the Batek population.
Female Foraging and Reproductive Success
In comparison with the relationship in males, the association between foraging performance and RS in females has received little attention. This is surprising given that female foraging contributes significantly to the food supply of hunter-gatherers (Endicott and Endicott 2008; Hill 1982; Lee 1968). Because female reproduction is limited mainly by resources (Hawkes 1996), it stands to reason that more efficient female foragers should achieve higher RS. Here we have presented the first direct test of the relationship between female huntergatherer foraging return rate and RS.
Female foraging return rate was uncorrelated with LRS for all resource types and categories when controlling for age (Fig. 1; Tables 1, S1). One potential explanation is that the Batek are not food limited, and that other factors, such as disease or status, govern survival and reproduction. This is unlikely, however, because acquiring food is difficult for human inhabitants of tropical rain forests (Headland 1987). In addition, female foraging involves direct trade-offs with childcare (Bliege Bird 1999; Hurtado et al. 1992), but we do not have data bearing on this trade-off. As noted by Hurtado et al. (1992), “the ecological causes of time allocation decisions cannot be adequately modeled without data on the long-term fitness benefits of spending time on different activities.”
Our findings also challenge the assumption that female foraging effort is solely directed toward family provisioning. It is often noted that men and women have different foraging goals with respect to trade-offs between social provisioning and attracting mates (Bird 1999; Hawkes 1996). But food acquired by Batek women is often widely distributed beyond immediate family members, much like meat obtained by male hunting. Whereas studies of male foraging have used such evidence to advance multiple working hypotheses to explain why men hunt (Gurven and Hill 2009; Smith 2004), there seems to be an implicit assumption that female foraging is unrelated to mate acquisition or social provisioning. This assumption is problematic because women are equally embedded in social networks that dictate survival and reproduction, and they almost certainly compete for high-quality males that have strong social support, provide childcare, or have good phenotypic qualities. In the Tsimane, however, work effort by women does not correlate with spousal status (von Rueden et al. 2010) and spouses tend to assort positively for work effort (Gurven and Hill 2009). Nevertheless, the data presented here suggest that alternative motivations for female foraging, such as social provisioning, deserve increased consideration.
Prosociality: Sharing and Cooperative Foraging
Prosociality is a plausible alternative determinant of RS among egalitarian Batek hunter-gatherers, where food is shared widely and overt status-seeking behavior is discouraged (Endicott and Endicott 2008). Yet we found that neither sharing proclivity nor tendency to cooperate during foraging was correlated with RS (Figs. 2, 3). Sharing and cooperative foraging were considered for all resource types and categories to test whether sharing of higher-quality resources such as meat specifically influenced RS (Tables 2, 3). Our results concord best with Tsimane data showing no correlation between the number of food-sharing partners and RS (von Rueden et al. 2010).
It is unlikely that data limitations explain our results. Figure S3 shows the relationship between the number of sample days and sharing or cooperative foraging metrics. Although bootstrapped 95% confidence intervals (CIs) revealed a significant amount of intra-individual variation, there was no strong effect of sample days on mean values of network metrics, and it appears that individuals with high sharing or cooperative foraging proclivity could be separated from those with a low proclivity based on nonoverlapping CIs (Fig. S3).
As with foraging performance, cultural norms in the Batek may preclude generous food sharers from deriving social benefits. For example, we documented numerous cases in which hunters brought meat back to camp, only to have that meat distributed by a different individual (in some cases, to the hut of the original hunter!). There are also no formal ceremonies or feasts to facilitate high-profile public sharing, and most sharing occurs between households with no fanfare. Similarly, cooperative foraging may play a relatively minor role in the social lives of the Batek, being just one example of a group activity. Finally, it is possible that we would be more likely to detect an effect of prosociality on proximate outcomes that might affect RS, such as status (von Rueden et al. 2008) or social support.
Kin Presence
Social support from kin plays an important role in small-scale societies. For example, the presence of living parents is associated with reduced child mortality in the Ache, and men with more living adult siblings experience increased age-specific fertility (Hill and Hurtado 1996). Kinship also structures the composition of cooperative foraging and hunting partnerships in the Efe, which are important for alliance building and social support (Bailey and Aunger 1989). In the Tsimane, the number of coresident consanguinal men is a significant predictor of male community-wide influence and the likelihood of success in physical confrontations (important aspects of status), and these relationships may define any direct association between the number of within-village consanguinal kin and male RS (von Rueden et al. 2010).
Yet we found no evidence that in-camp kin presence predicts LRS in the Batek. This outcome is not due to a lack of variation in either LRS or our measures of kin presence. Other forms of social support, such as friendship, may be more important in the Batek, as demonstrated by the extension of kin terms to unrelated individuals of similar age/ sex classes despite awareness of true genetic relationships (Endicott and Endicott 2008). Further, the timespan of our study may not capture a lifetime metric of kin presence, especially for younger individuals who have not yet married.
What Can Studies of Reproductive Success Tell Us about Human Behavior and Evolution?
Linking behavior and RS is vital for measuring selection in progress, identifying the adaptive significance of phenotypic traits, validating fitness proxies (Smith 1979; Stephens and Krebs 1986), and generating a referential model for investigating the dynamics and causal factors underlying evolutionary processes that are visible in the fossil and archaeological records. Ultimately, we consider studies of RS in humans to be a productive avenue of research, but with two major caveats. First, the inability to detect trends between proximate factors and RS should not be interpreted as definitive evidence that no such relationship exists. Sample sizes in studies of small-scale societies are often small, it is difficult to obtain reliable estimates of predictor variables that overcome measurement error (Hill and Kintigh 2009) and indicate performance across the whole lifespan, and RS can be influenced by numerous variables. Large effect sizes and comparative evidence are necessary to build a generalized theory of the determinants of RS. Second, LRS should not be used to replace proximate fitness measures such as daily energetic costs or food acquisition. Instead, fitness proxies and RS should be considered within a single unified framework that takes advantage of techniques (such as structural equation modeling) that enable researchers to build and compare alternative models representing pathways to RS.
[Back Matter]
Acknowledgments
We thank all the Batek who have generously participated in our collective research over the years. We would also like to thank Karen Endicott for help in collecting data, Aya Kawai for sharing information on Batek kinship and genealogy, and Lye Tuck-Po and Thomas Overly for advice and assistance. Kristen Hawkes, Mark McPeek, and several anonymous reviewers provided invaluable feedback on earlier drafts.
This work was supported by the Wenner-Gren Foundation (grant num. 8551 to IT), National Science Foundation (Graduate Research Fellowships to TSK and VVV; DDRIG to TSK), National Geographic Society (Young Explorer’s Grant to TSK), Leakey Foundation (Research Grant to TSK), and the Claire Garber Goodman Fund at Dartmouth College. The Endicotts’ research was supported by the National Institute of Mental Health, University of Malaya, Australian National University, Fulbright-Hays Foundation, American Council of Learned Societies, and Social Science Research Council.
This research was approved by Dartmouth College’s Committee for the Protection of Human Subjects (protocol #22410) and was conducted with full approval and support of the Malaysian government and Jabatan Hal Ehwal Orang Asli (formerly Department of Aboriginal Affairs) under permits VC/60050/70; #045847; 581/70, VC/60050; #147485, VC/60050; #4227, VC/60050; 674/90 (KME), UPE: 40/200/19/2029, UPE: 40/200/19/2889, and JPHL&TN (IP) 80-4/2 Jilid (IT).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aghakhanian, F., Yunus, Y., Naidu, R., Jinam, T., Manica, A., Hoh, B. P., & Phipps, M. E. (2015). Unravelling the genetic history of negritos and indigenous populations of Southeast Asia. Genome Biology and Evolution, 7(5), 1206–1215.
Alvard, M. S. (2003). Kinship, lineage, and an evolutionary perspective on cooperative hunting groups in Indonesia. Human Nature, 14(2), 129–163.
Alvard, M. S., & Gillespie, A. (2004). Good Lamalera whale hunters accrue reproductive benefits. Research in Economic Anthropology, 23(4), 223–245.
Apicella, C. L. (2014). Upper-body strength predicts hunting reputation and reproductive success in Hadza hunter-gatherers. Evolution and Human Behavior, 35(6), 508–518.
Apicella, C. L., Marlowe, F. W., Fowler, J. H., & Christakis, N. A. (2012). Social networks and cooperation in hunter-gatherers. Nature, 481(7382), 497–501.
Bailey, R. C. (1991). The behavioral ecology of Efe pygmy men in the Ituri Forest, Zaire. Ann Arbor: University of Michigan Museum.
Bailey, R. C., & Aunger, R. (1989). Significance of the social relationships of Efe pygmy men in the Ituri Forest, Zaire. American Journal of Physical Anthropology, 78(4), 495–507.
Benjamin, G. (1976). Austroasiatic subgroupings and prehistory in the Malay Peninsula. Oceanic Linguistics Special Publication, 13, 37–128.
Bliege Bird, R. (1999). Cooperation and conflict: the behavioral ecology of the sexual division of labor. Evolutionary Anthropology, 8(2), 65–75.
Bliege Bird, R., Smith, E. A., & Bird, D. W. (2001). The hunting handicap: costly signaling in human foraging strategies. Behavioral Ecology and Sociobiology, 50(1), 9–19.
Bonacich, P., & Lloyd, P. (2001). Eigenvector-like measures of centrality for asymmetric relations. Social Networks, 23(3), 191–201.
Cameron, A. C., & Trivedi, P. K. (1990). Regression-based tests for overdispersion in the Poisson model. Journal of Econometrics, 46(3), 347–364.
Campbell, K. L., & Wood, J. W. (1988). Fertility in traditional societies. In Natural human fertility: Social and biological determinants (pp. 39–69). Basingstoke: Macmillan Press.
Cashdan, E. A. (1980). Egalitarianism among hunters and gatherers. American Anthropologist, 82(1), 116–120.
Chudek, M., & Henrich, J. (2011). Culture-gene coevolution, norm-psychology and the emergence of human prosociality. Trends in Cognitive Sciences, 15(5), 218–226.
Clutton-Brock, T. H. (1988). Reproductive success: Studies of individual variation in contrasting breeding systems. Chicago: University of Chicago Press.
Crognier, E. (2003). Reproductive success: which meaning? American Journal of Human Biology, 15, 352–360.
Csardi, G., & Nepusz, T. (2006). The igraph software package for complex network research. InterJournal, Complex Systems, 1695, 1–9.
Dart, R. A. (1953). The predatory transition from ape to man. International Anthropological and Linguistic Review, 1, 201–218.
Dunn, F. (1975). Rain-forest collectors and traders: A study of resource utilization in modern and ancient Malaya (Vol. 5). Kuala Lumpur: The Malaysian Branch of the Royal Asiatic Society.
Dwyer, P. D., & Minnegal, M. (1993). Are Kubo hunters “show offs”? Ethology and Sociobiology, 14(1), 53–70.
Endicott, K. M. (1979). The hunting methods of the Batek Negritos of Malaysia: A problem of alternatives. Canberra Anthropology, 2(2), 7–22.
Endicott, K. M. (1984). The economy of the Batek of Malaysia: annual and historical perspectives. Research in Economic Anthropology, 6, 29–52.
Endicott, K. M. (2011). Cooperative autonomy: Social solidarity among the Batek of Malaysia. In T. Gibson & K. Sillander (Eds.), Anarchic solidarity: Authority, equality, and fellowship in Southeast Asia (pp. 62–87). New Haven: Yale University Council on Southeast Asia Studies.
Endicott, P. (2013). Revisiting the “negrito” hypothesis: A transdisciplinary approach to human prehistory in Southeast Asia. Human Biology, 85(1–3), 7–20.
Endicott, K. M., & Endicott, K. L. (2008). The headman was a woman: The gender egalitarian Batek of Malaysia. Long Grove: Waveland Press.
Endicott, K. L., & Endicott, K. M. (2014). Batek childrearing and morality. In D. Narvaez, A. Fuentes, & P. Gray (Eds.), Ancestral landscapes in human evolution: Culture, childrearing and social wellbeing (pp. 108–125). New York: Oxford University Press.
Gurven, M. (2004). To give and to give not: The behavioral ecology of human food transfers. Behavioral and Brain Sciences, 27, 543–559.
Gurven, M., & Hill, K. (2009). Why do men hunt? A reevaluation of “Man the Hunter” and the sexual division of labor. Current Anthropology, 50(1), 51–74.
Gurven, M., & von Rueden, C. (2006). Hunting, social status and biological fitness. Social Biology, 53(1–2), 81–99.
Gurven, M., Hill, K., Kaplan, H. S., Hurtado, A., & Lyles, R. (2000). Food transfers among Hiwi foragers of Venezuela: tests of reciprocity. Human Ecology, 28(2), 171–218.
Gurven, M., Allen-Arave, W., Hill, K., & Hurtado, A. M. (2001). Reservation food sharing among the Ache of Paraguay. Human Nature, 12, 273–297.
Gurven, M., von Rueden, C., Stieglitz, J., Kaplan, H. S., & Rodriguez, D. E. (2014). The evolutionary fitness of personality traits in a small-scale subsistence society. Evolution and Human Behavior, 35(1), 17–25.
Handcock, M. S., Hunter, D. R., Butts, C. T., Goodreau, S. M., & Morris, M. (2008). statnet: Software tools for the representation, visualization, analysis and simulation of network data. Journal of Statistical Software, 24(1), 1548–7660.
Hansen, M. C., Potapov, P. V., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A., et al. (2013). Highresolution global maps of 21st-century forest cover change. Science, 342(6160), 850–853.
Hawkes, K. (1996). Foraging differences between men and women. In James Steele and Stephen Shennan (Eds.), Power, sex and tradition: The archaeology of human ancestry (pp. 283–305). London: Routledge.
Hawkes, K., & Bliege Bird, R. (2002). Showing off, handicap signaling, and the evolution of men’s work. Evolutionary Anthropology, 11(2), 58–67.
Hawkes, K., O’Connell, J. F., & Blurton Jones, N. G. (2001). Hunting and nuclear families: some lessons from the Hadza about men’s work. Current Anthropology, 42(5), 681–709.
Headland, T. N. (1987). The wild yam question: how well could independent hunter-gatherers live in a tropical rain forest ecosystem? Human Ecology, 15(4), 463–491.
Hewlett, B. S. (1988). Sexual selection and paternal investment among Aka pygmies. In L. Betzig, M. B. Mulder, & P. Turke (Eds.), Human reproductive behavior (pp. 263–276). Cambridge: Cambridge University Press.
Hill, K. (1982). Hunting and human evolution. Journal of Human Evolution, 11(6), 521–544.
Hill, K., & Hurtado, A. M. (1996). Ache life history: The ecology and demography of a foraging people. New York: Aldine/Transaction.
Hill, K., & Kintigh, K. (2009). Can anthropologists distinguish good and poor hunters? Implications for hunting hypotheses, sharing conventions, and cultural transmission. Current Anthropology, 50(3), 369–378.
Hill, K., Walker, R. S., Bozicevic, M., Eder, J., Headland, T., Hewlett, B., et al. (2011). Coresidence patterns in hunter-gatherer societies show unique human social structure. Science, 331(6022), 1286–1289.
Hurtado, A. M., Hill, K., Hurtado, I., & Kaplan, H. S. (1992). Trade-offs between female food acquisition and child care among Hiwi and Ache foragers. Human Nature, 3(3), 185–216.
Jaeggi, A. V., & Gurven, M. (2013). Reciprocity explains food sharing in humans and other primates independent of kin selection and tolerated scrounging: a phylogenetic meta-analysis. Proceedings of the Royal Society of London B: Biological Sciences, 280(1768), 20131615.
Kelly, R. L. (2013). The lifeways of hunter-gatherers: The foraging spectrum. Cambridge: Cambridge University Press.
Kent, S. (1996). Hunting variability at a recently sedentary Kalahari village. In S. Kent (Ed.), Cultural diversity among twentieth-century foragers: An African perspective (pp. 125–156). Cambridge: Cambridge University Press.
Kirchengast, S. (2000). Differential reproductive success and body size in !Kung San people from northern Namibia. Collegium Antropologicum, 24(1), 121–132.
Lee, R. B. (1968). What hunters do for a living, or, how to make out on scarce resources. In R. B. Lee & I. DeVore (Eds.), Man the hunter (pp. 30–48). Chicago: Aldine.
Lee, R. B. (1969). Eating Christmas in the Kalahari. Natural History, 78(10), 14–22.
Lee, R. B. (2003). The Dobe Ju/’hoansi (3rd ed.). Belmont, CA: Wadsworth.
Lye, T.-P. (1997). Knowledge, forest, and hunter-gatherer movement: The Batek of Pahang, Malaysia. PhD dissertation, University of Hawai’i.
Lye, T.-P. (2004). Changing pathways: Forest degradation and the Batek of Pahang, Malaysia. Lanham: Lexington Books.
Marlowe, F. (2000). The patriarch hypothesis: an alternative explanation of menopause. Human Nature, 11(1), 27–42.
Nolin, D. A. (2010). Food-sharing networks in Lamalera, Indonesia: reciprocity, kinship, and distance. Human Nature, 21(3), 243–268.
Nolin, D. A. (2011). Kin preference and partner choice: patrilineal descent and biological kinship in Lamaleran cooperative relationships. Human Nature, 22(1–2), 156–176.
R Core Team. (2015). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Sear, R., & Mace, R. (2008). Who keeps children alive? A review of the effects of kin on child survival. Evolution and Human Behavior, 29(1), 1–18.
Sherman, P. W., & Reeve, H. K. (1997). Forward and backward: alternative approaches to studying human social evolution. In L. Betzig (Ed.), Human nature: a critical reader (pp. 147–158). New York: Oxford University Press.
Singh, M. (2018). The cultural evolution of shamanism. Behavioral and Brain Sciences, 41, e66.
Smith, E. A. (1979). Human adaptation and energetic efficiency. Human Ecology, 7(1), 53–74.
Smith, E. A. (2004). Why do good hunters have higher reproductive success? Human Nature, 15(4), 343–364.
Smith, E. A., & Bliege Bird, R. L. (2000). Turtle hunting and tombstone opening: public generosity as costly signaling. Evolution and Human Behavior, 21(4), 245–261.
Smith, E. A., Mulder, M. B., & Hill, K. (2001). Controversies in the evolutionary social sciences: a guide for the perplexed. Trends in Ecology & Evolution, 16(3), 128–135.
Smith, E. A., Bliege Bird, R., & Bird, D. W. (2003). The benefits of costly signaling: Meriam turtle hunters. Behavioral Ecology, 14(1), 116–126.
Smith, E. A., Hill, K., Marlowe, F., Nolin, D., Wiessner, P., Gurven, M., et al. (2010). Wealth transmission and inequality among hunter-gatherers. Current Anthropology, 51(1), 19.
Smith, D., Schlaepfer, P., Major, K., Dyble, M., Page, A. E., Thompson, J., et al. (2017). Cooperation and the evolution of hunter-gatherer storytelling. Nature Communications, 8, 1853.
Sodhi, N. S., Koh, L. P., Brook, B. W., & Ng, P. K. L. (2004). Southeast Asian biodiversity: an impending disaster. Trends in Ecology & Evolution, 19(12), 654–660.
Stephens, D. W., & Krebs, J. R. (1986). Foraging theory. Princeton: Princeton University Press.
Suratman, M., Dain, M., Singh, H., & Ismail, N. (2012). Taman Negara: Towards biodiversity conservation and sustainability. Selangor Darul Ehsan: Universiti Teknologi MARA, Penerbit Press.
Symons, D. (1989). A critique of Darwinian anthropology. Ethology and Sociobiology, 10(1), 131–144.
Venkataraman, V. V., Kraft, T. S., Dominy, N. J., & Endicott, K. M. (2017). Hunter-gatherer residential mobility and the marginal value of rainforest patches. Proceedings of the National Academy of Sciences, 114(12), 3097–3102.
von Rueden, C., & Jaeggi, A. V. (2016). Men’s status and reproductive success in 33 nonindustrial societies: Effects of subsistence, marriage system, and reproductive strategy. Proceedings of the National Academy of Sciences USA, 113(39), 10824–10829.
von Rueden, C., Gurven, M., & Kaplan, H. S. (2008). The multiple dimensions of male social status in an Amazonian society. Evolution and Human Behavior, 29(6), 402–415.
von Rueden, C., Gurven, M., & Kaplan, H. S. (2010). Why do men seek status? Fitness payoffs to dominance and prestige. Proceedings of the Royal Society B: Biological Sciences, 278, 2223–2232.
von Rueden, C., Lukaszewski, A. W., & Gurven, M. (2015). Adaptive personality calibration in a human society: effects of embodied capital on prosocial traits. Behavioral Ecology, 26(4), 1071–1082.
Washburn, S., & Lancaster, C. (1968). The evolution of hunting. In R. B. Lee & I. DeVore (Eds.), Man the hunter (pp. 293–303). Chicago: Aldine.
Wiessner, P. (2002). Hunting, healing, and hxaro exchange: a long-term perspective on !Kung (Ju/’hoansi) large-game hunting. Evolution and Human Behavior, 23(6), 407–436.
Woodburn, J. (1982). Egalitarian societies. Man, 17, 431–451.
Ziker, J. P. (2014). Sharing, subsistence, and social norms in Northern Siberia. In J. Ensminger & J. Henrich (Eds.), Experimenting with social norms: Fairness and punishment in cross-cultural perspective (pp. 337–356). New York: Russell Sage Foundation.
Ziker, J. P., Nolin, D. A., & Rasmussen, J. (2016). The effects of wealth on male reproduction among monogamous hunter-fisher-trappers in Northern Siberia. Current Anthropology, 57(2), 221–229.
[About the Authors]
Thomas S. Kraft obtained his PhD from Dartmouth College and is now a postdoctoral scholar at the University of California, Santa Barbara. He is a human behavioral ecologist with a focus on social organization, behavior, cooperation, and health. He has conducted fieldwork with several subsistence populations that occupy rainforest habitats, including the Batek of Malaysia and the Tsimane of Bolivia.
Vivek V. Venkataraman earned his PhD from Dartmouth College and is presently a research fellow at the Institute for Advanced Study in Toulouse, France, and a research associate in the Department of Human Evolutionary Biology at Harvard University. He is a behavioral ecologist interested in the evolution of the human diet, with a current focus on the ecology and energetics of human subsistence strategies.
Ivan Tacey is currently a postdoctoral research associate at the Exeter Anthrozoology as Symbiotics Ethics Working Group (EASE) at the University of Exeter in the UK. He has recently submitted his doctoral thesis to the University of Helsinki in Finland. Since 2007, he has worked with the Batek and Jahai hunter-gatherers of the Malay Peninsula. His research has focused on how globalization and marginalization have led to realignments of indigenous peoples’ religions, claims to places, and environmental interactions. He is a member of the Scientific Committee and Editorial Board for the International Society for Academic Research on Shamanism (ISARS).
Nathaniel J. Dominy is the Charles Hansen Professor of Anthropology at Dartmouth College. He studies the behavior, ecology, and functional morphology of humans and nonhuman primates. Much of his work entails tropical fieldwork in tandem with mechanical, molecular, and stable isotope analyses. His overarching goal is to better understand how and why adaptive shifts occurred during primate evolution.
Kirk M. Endicott has a PhD from Harvard University and a DPhil from the University of Oxford. He taught at several universities and retired in 2011 after teaching for 29 years at Dartmouth College, where he is now a professor emeritus. He is a sociocultural anthropologist with special interest in hunter-gatherer economies, social organization and behavior, gender relations, and religions, and in human rights of indigenous peoples. He did fieldwork with the Batek and other Malaysian indigenous minority groups intermittently between 1971 and 2004. He continues to collaborate with colleagues studying the relevance of Batek studies for the understanding of human evolution.
Affiliations
Thomas S. Kraft1,2, Vivek V. Venkataraman3, Ivan Tacey4, Nathaniel J. Dominy1 & Kirk M. Endicott1
1 Department of Anthropology, Dartmouth College, Hanover, NH 03755, USA
2 Present address: Department of Anthropology, University of California-Santa Barbara, Santa Barbara, CA 93106, USA
3 Department of Human Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA
4 Exeter Anthrozoology as Symbiotics Ethics Working Group, University of Exeter, Exeter, UK
[1] By standard meta-analytical practice the estimate in von Rueden and Jaeggi ([2016]) is reported as Fisher’s Z (Zr) and was transformed here to a standard correlation coefficient.
{1} Was the measure used for hunting ability continuous?
{2} For analysis of fertility as response variable.
{3} Was there a significant correlation between hunting and fertility?