Previous address: Department of Ecology, Evolution, and Behavior, University of Minnesota, Saint Paul, MN 55108, USA.
Jeffrey R. Stevens
The selfish nature of generosity
harassment and food sharing in primates
(b) Experimental apparatus and treatments
(a) Non-contingent benefits of harassment
(b) Harassment effects on sharing
Animals may share food to gain immediate or delayed fitness benefits. Previous studies of sharing have concentrated on delayed benefits such as reciprocity, trade and punishment. This study tests an alternative model (the harassment or sharing-under-pressure hypothesis) in which a food owner immediately benefits because sharing avoids costly harassment from a beggar. I present an experiment that varies the potential ability of the beggar to harass, and of the owner to defend the food, to examine the effects of harassment on food sharing in two primate species: chimpanzees (Pan troglodytes) and squirrel monkeys (Saimiri boliviensis). For both species, high levels of harassment potential significantly increased both beggar harassment and sharing by the owner. Food defensibility did not affect harassment or sharing. Interestingly, squirrel monkeys and chimpanzees shared equally frequently with conspecifics despite a much higher natural sharing rate in chimpanzees. These results suggest that harassment can play a significant role in primate food sharing, providing a simple alternative to reciprocity. The selfish nature of harassment has implications for economic, psychological and evolutionary studies of cooperative systems.
Keywords: cooperation; food sharing; harassment; primates; punishment
Introduction
Historically, studies of altruism have focused on kin selection (Hamilton 1964), reciprocal altruism (Trivers 1971) and trade (Sahlins 1972) as potential explanations of generosity. Recently, however, exciting new theoretical and empirical studies have investigated the role of sanctions in maintaining generosity (Clutton-Brock & Parker 1995; Fehr & Ga¨chter 2000; Andreoni et al. 2003). Sanctions involve imposing costs on a defector following a selfish act under the assumption that, eventually, the defector will act altruistically to avoid sanctions. Sanctions can be categorized either as harassment or punishment, depending on whether the costs are imposed during or following defection. Because punishment follows a selfish act, it poses two interesting problems. First, punishment is costly to the punisher. If the punisher does not interact with the defector again, it pays the cost of punishing while others may benefit from the defector switching to cooperation, so punishing itself may be altruistic. Therefore, a temptation to avoid punishing exists, and another level of punishment is needed to punish those that do not altruistically punish (Boyd & Richerson 1992). To prevent this infinite recursion, researchers have proposed cost-free punishment (Hirshleifer & Rasmusen 1989), group selection (Gintis 2000; Boyd et al. 2003) and transmission of conformity (Henrich & Boyd 2001) as mechanisms to maintain punishment. Second, even if the punisher does receive selfish benefits for punishing, these benefits occur only after the punishment, so the punisher pays an immediate cost for a future benefit (Boyd & Richerson 1992; Clutton-Brock & Parker 1995). Because the timing of costs and benefits plays a crucial role in the evolution of cooperation (Mesterton-Gibbons & Dugatkin 1997; Stevens & Gilby 2004), waiting to recoup the benefits until defectors ‘learn’ to be generous may be difficult for punishers, particularly if they discount future rewards. However, imposing sanctions during the selfish act (harassment) eliminates both problems with punishment. When an animal harasses, it may manipulate the defector into being generous, thereby providing an immediate benefit to both individuals. Harassment, then, may provide a selfish explanation of apparently altruistic behaviour.
The effect of harassment on generosity may account for many instances of animal food sharing. Field observations of chimpanzees suggest that persistent begging can force food owners to avoid beggars or cause them to share food (Teleki 1973; Goodall 1986). Wrangham (1975) predicted that the energetic and opportunity costs of defending food might force owners to ‘pay’ beggars with part of the food to avoid harassment (the ‘sharing-underpressure’ hypothesis). Later, Blurton Jones (1984) proposed the ‘tolerated theft’ model of sharing, predicting that satiation decreases the marginal fitness value of the remaining resource. The owner should share to avoid costs associated with defending the food, because the owner values the remaining food less than a hungry newcomer. Stevens & Stephens (2002) have extended this idea by considering how beggar harassment may impose a cost on food owners. The model predicts that beggars should harass only when they can offset their own cost of harassing by gaining ‘non-contingent benefits’ defined as benefits other than shared food (e.g. dropped scraps or stolen pieces). Thus, harassment is profitable only when the owner cannot completely defend the food. If the harassment costs imposed on the owner are high enough, it may benefit more by sharing part of the food rather than accepting the costs. Therefore, sharing may provide selfish, immediate benefits to the sharer by reducing the costs of defence.
Observational and experimental studies have demonstrated food sharing in a number of species, including insects, birds, bats, cetaceans, small mammals and primates (Feistner & McGrew 1989; Stevens & Gilby 2004). Many of these studies investigate reciprocity as a possible explanation of sharing (Brosnan & de Waal 2002; Hauser et al. 2003). Few studies, however, empirically test the importance of harassment on food sharing. This study examines harassment by testing two predictions from the Stevens and Stephens model: (i) beggars should preferentially harass when they can gain non-contingent benefits; and (ii) owners should share more food when harassed. To test these predictions, I manipulated food divisibility and the beggar’s ability to harass. By manipulating food divisibility, I controlled the owner’s ability to defend the food and, therefore, the non-contingent benefits. Previous work has demonstrated that chimpanzees give more foodelicited calls (often associated with sharing food with others) when food is divisible (Hauser et al. 1993). I manipulated the harassment costs inflicted on the owner by allowing the beggar to harass or by preventing harassment with a mesh partition. Table 1 summarizes the predicted behaviour for both owners and beggars in each of the experimental treatments.
To evaluate the generality of harassment, I conducted experiments on two species of captive primates: common chimpanzees (Pan troglodytes) and squirrel monkeys (Saimiri boliviensis). I used these two species because they differ in their natural sharing rates. Chimpanzees frequently share in both natural and captive situations (Goodall 1986; de Waal 1989), whereas squirrel monkeys do not often share food (Fragaszy & Mason 1983). If both species exhibit effects of harassment on sharing, this strongly supports the importance of selfish explanations of food sharing.
Table 1. Predicted responses by food owners and beggars to Partition and Divisibility treatments.
(Experimental results support all predictions except beggar response in Partition Present/Divided food treatment and owner response in Partition Absent/Solid food treatment.)
| solid food | divided food | |
| Partition Present | ||
| beggar | not harass | harassa |
| owner | defend | defend |
| Partition Absent | ||
| beggar | not harass | harass |
| owner | defendb | share |
a Squirrel monkeys did not harass.
b Neither chimpanzees nor squirrel monkeys defended.
Material and Methods
(a) Subjects
I tested common chimpanzees (P. troglodytes) at the Southwest Foundation for Biomedical Research in San Antonio, Texas, from May to August 2001. Subjects included 12 adult females (ages ranged from 17 to 41 years old), paired within their social groups (three to five members). I paired unrelated individuals of similar age, dominance rank and food motivation to minimize competitive asymmetries. I tested Bolivian squirrel monkeys (S. boliviensis) from the Squirrel Monkey Breeding and Research Resource at the University of South Alabama in Mobile, Alabama, from September to November 2001. Again, subjects included 12 unrelated adult females (ages ranged from 4 to 9 years old), paired within their social groups (20–37 members). All pairs remained constant throughout the experiment.
(b) Experimental apparatus and treatments
I tested the chimpanzees in adjacent outdoor cages; each measuring 3.15 m × 4.56 m × 2.88 m. Metal fencing (4.8 cm × 4.8 cm square holes) enclosed most of the cage, except for the concrete floor and back wall. Hardware cloth covered the fencing separating the two adjacent experimental cages (1.4 cm ×1.4 cm square holes). A 142 cm × 50 cm section of the 4.8 cm ×4.8 cm square hole fencing was exposed, allowing the chimpanzees to interact through the mesh.
The squirrel monkeys were tested in a 155 cm × 64 cm × 77 cm Allentown cage, composed of stainless steel mesh (2.3 cm × 4.3 cm rectangular holes) on all sides. A Plexiglas sheet provided a solid floor for the cage. The 65 cm × 71 cm partition separating the two chambers consisted of Gard’n Fence plastic mesh (3.5 cm × 5.5 cm rectangular holes).
The experimental treatments followed a 2 × 2 repeated-measures design, including partition and food divisibility as factors. Partition treatments manipulated the subjects’ potential to harass each other by varying the presence or absence of a mesh partition. When present, the mesh partition limited access between the subjects by separating them in adjacent cages. When absent, the subjects had full access to both cages and to one another.
The second treatment factor varied the defensibility of the food source by manipulating whether the food was either solid or partly divided (Divisibility treatment). Owners could monopolize solid food because it remained intact as they consumed it. Partly divided food (food that was cut multiple times but not completely through) disintegrated as the owner consumed it, thereby reducing the monopolizability of the food. I used three bananas (a total of ca. 600 g) as food for chimpanzees and a 20 g cube of cantaloupe for squirrel monkeys.
Each subject experienced the factorial combination of these four treatment levels as both owner and beggar (non-owner). I conducted four replicates of these randomly ordered treatment combinations for a total of 32 trials per pair (2 partition levels × 2 divisibility levels × 2 ownership levels × 4 replicates). Each pair experienced one trial per day in a randomized test order until completing all trials. Trials occurred between 08.00 and 14.00 before regular daily feedings.
(c) Experimental procedure
Trials ran slightly differently for the two species. For chimpanzees, I placed the solid or partly divided bananas on the floor ca. 20 cm from the mesh partition. I transferred the randomly chosen owner into the cage with food and the beggar into the adjacent cage without food. For Partition Absent trials, I opened the door separating the two experimental cages when the owner possessed the food. The door remained closed for Partition Present trials.
For squirrel monkeys, I placed the randomly chosen owner into the left chamber of the experimental cage and the beggar in the right chamber. I placed the solid or partly divided cantaloupe in the owner’s food dish. This configuration situated the food near the beggar but out of her reach (10 cm from the partition). I removed the entire partition separating the chambers upon possession of the cantaloupe in Partition Absent trials and left the partition in place for Partition Present trials.
I operationally defined sharing and harassment loosely based on de Waal (1997). Specifically the following.
Sharing events:
(i) collect near—beggar recovers food from within arm’sreach of owner;
(ii) relaxed claim—beggar takes part of food that owner possesses without resistance from owner;
(iii) food giving—owner facilitates transfer of food by activelymoving it toward beggar.
Harassment events:
(i) forced claim—beggar forcefully takes food from owner;owner resists;
(ii) unsuccessful forced claim—beggar attempts but fails totake food from owner;
(iii) passive begging—beggar sits near and stares at owner butno physical contact;
(iv) active begging—beggar physically contacts owner or food;(v) attack—beggar aggressively interacts with owner.
I recorded the total number of harassment events (harassment frequency), the total time spent harassing (harassment duration), the total number of sharing events (sharing frequency), the proportion of food shared by owner (proportion shared) and the proportion of food consumed by each subject (proportion consumed) for each trial. The onset of harassment duration started at the first instance of a harassment event and continued until no harassment events were observed for 5 s. All of these measures were scored from videotape analysis. The proportion shared and proportion consumed variables were arcsine, square-root transformed for all analyses to provide more normally distributed data (Zar 1999).
Results
(a) Non-contingent benefits of harassment
The Divisibility treatment tested whether possible noncontingent benefits (divided food) increased harassment by beggars. Repeated-measures analyses of variance indicate that the divisibility treatment significantly influenced chimpanzee harassment frequency (F1,11 = 7.17, p = 0.0215) but not squirrel monkey harassment frequency (F1,11 = 0.75, p = 0.4036). Therefore, chimpanzee beggars responded to divided food by increasing their harassment, but squirrel monkeys did not (figure 1a). Another measure of non-contingent benefits is whether beggars consume more food when they harass but owners do not share. To test this, I conducted stepwise regressions on the effects of harassment frequency and duration on beggar’s consumption amount in No Partition trials in which no sharing occurred. Squirrel monkey beggars consumed more food (in the absence of sharing) when they harassed more frequently (F1,56 = 7.78, p = 0.0072), but chimpanzee beggars did not. These results suggest that the Divisibility treatment did manipulate non-contingent benefits for the chimpanzees but not for the squirrel monkeys. The squirrel monkeys, however, reaped benefits by consuming more when harassing.
(b) Harassment effects on sharing
Repeated-measures ANOVAs indicate that more harassment occurred in the No Partition treatment. As predicted, the chimpanzees harassed more frequently (F1,11 = 11.24, p = 0.0064) and for longer durations (F1,11 = 12.09, p = 0.0052) in the absence of a partition (figure 1a). Squirrel monkeys showed similar effects (harassment frequency: F1,11 = 24.43, p = 0.0004; harassment duration: F1,11 = 12.74, p = 0.0044). Harassment frequency and duration were positively correlated for both species, indicating that longer harassment bouts did not simply spread out the harassment events (chimpanzees: r = 0.65, p 0.0001; squirrel monkeys: r = 0.65, p 0.0001).
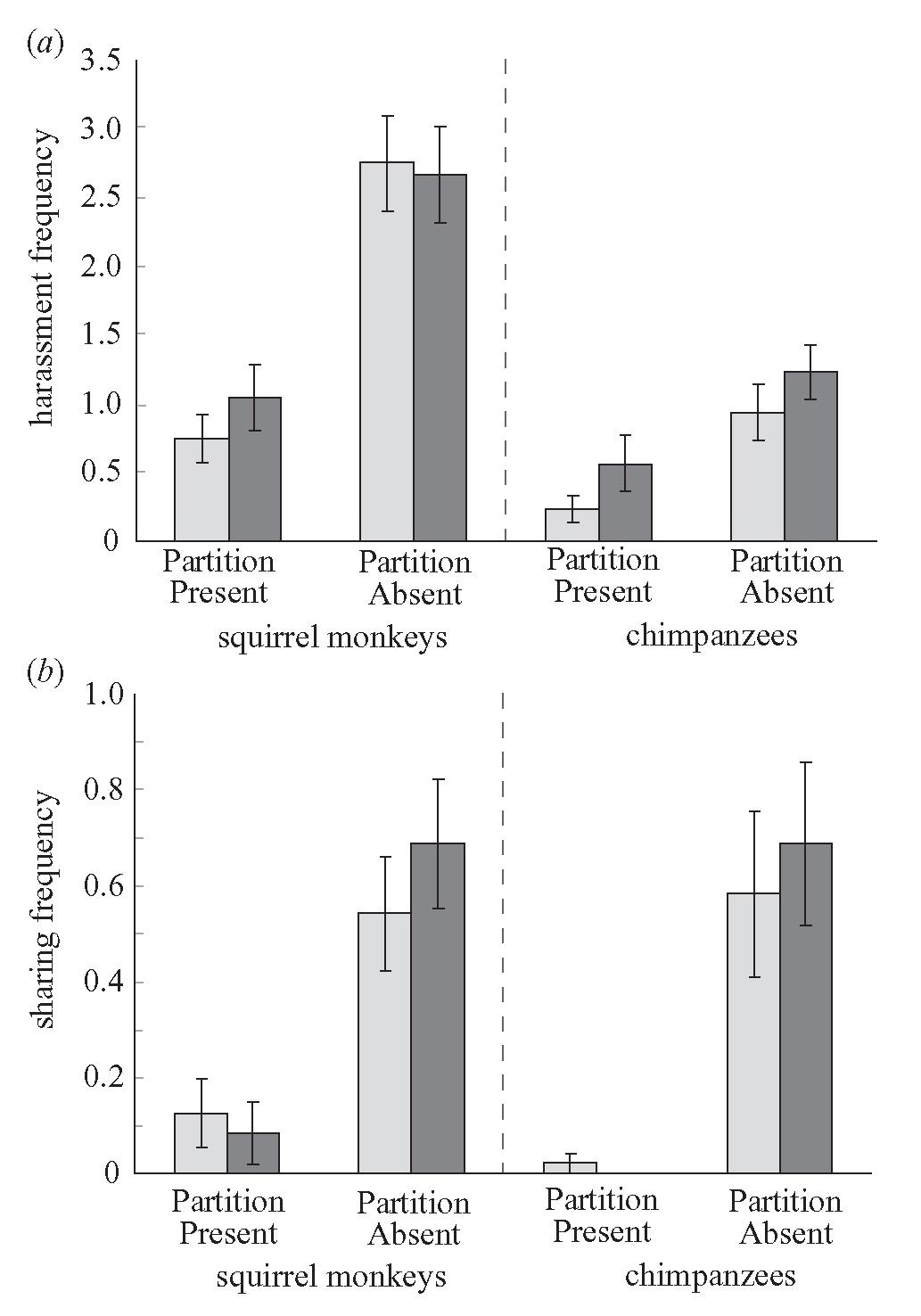
Given that the Partition treatment changed beggar harassment levels, I tested whether this treatment also influenced sharing by the owner. The partition influenced sharing frequency (chimpanzees: F1,11 = 10.43, p = 0.0080; squirrel monkeys: F1,11 = 15.96, p = 0.0021) and proportion of food shared (chimpanzees: F1,11 = 5.38, p = 0.0406; squirrel monkeys: F1,11 = 13.63, p = 0.0035). Thus, more sharing occurred in the absence of the partition and in the face of more harassment (figure 1b).
To ensure that harassment itself influenced sharing, I examined harassment and sharing in the absence of a partition. In fact, sharing did occur more frequently when beggars harassed than when they did not for both chimpanzees (t94 = 3.80, p = 0.0003) and squirrel monkeys (t94 = 2.95, p = 0.004). Stepwise multiple regressions testing the effects of frequency and duration of harassment on sharing indicate that the frequency of sharing events increased continuously with harassment frequency in both chimpanzees (F1,94 = 15.77, p = 0.0001) and squirrel monkeys (F1,94 = 5.39, p = 0.02). Also, the proportion of food shared increased linearly with harassment duration (chimpanzees: F1,94 = 8.51, p = 0.004; squirrel monkeys: F1,94 = 5.17, p = 0.03). These results indicate that harassment from the beggar did increase her chance of receiving food, and more intense harassment elicited more frequent and larger amounts of sharing.
In establishing pairs for this experiment, I chose individuals of similar rank. A few of the pairs, however, did have relative dominance differences (four chimpanzee pairs and three squirrel monkey pairs). Although the degree of dominance asymmetry was small among these pairs, I tested the effect of relative dominance on both species. Dominance did not affect harassment (chimpanzees: t126 =0.43, p = 0.67; squirrel monkeys: t94 = 1.28, p = 0.20) or sharing in either species (chimpanzees: t126 =0.52, p = 0.61; squirrel monkeys: t94 =0.77, p = 0.44). Therefore, my pattern of results cannot be explained by dominant individuals harassing subordinates to share. Regardless of whether there are dominance effects, the within-subjects design of this experiment demonstrates that harassment influences sharing within an individual.
(c) Comparative results
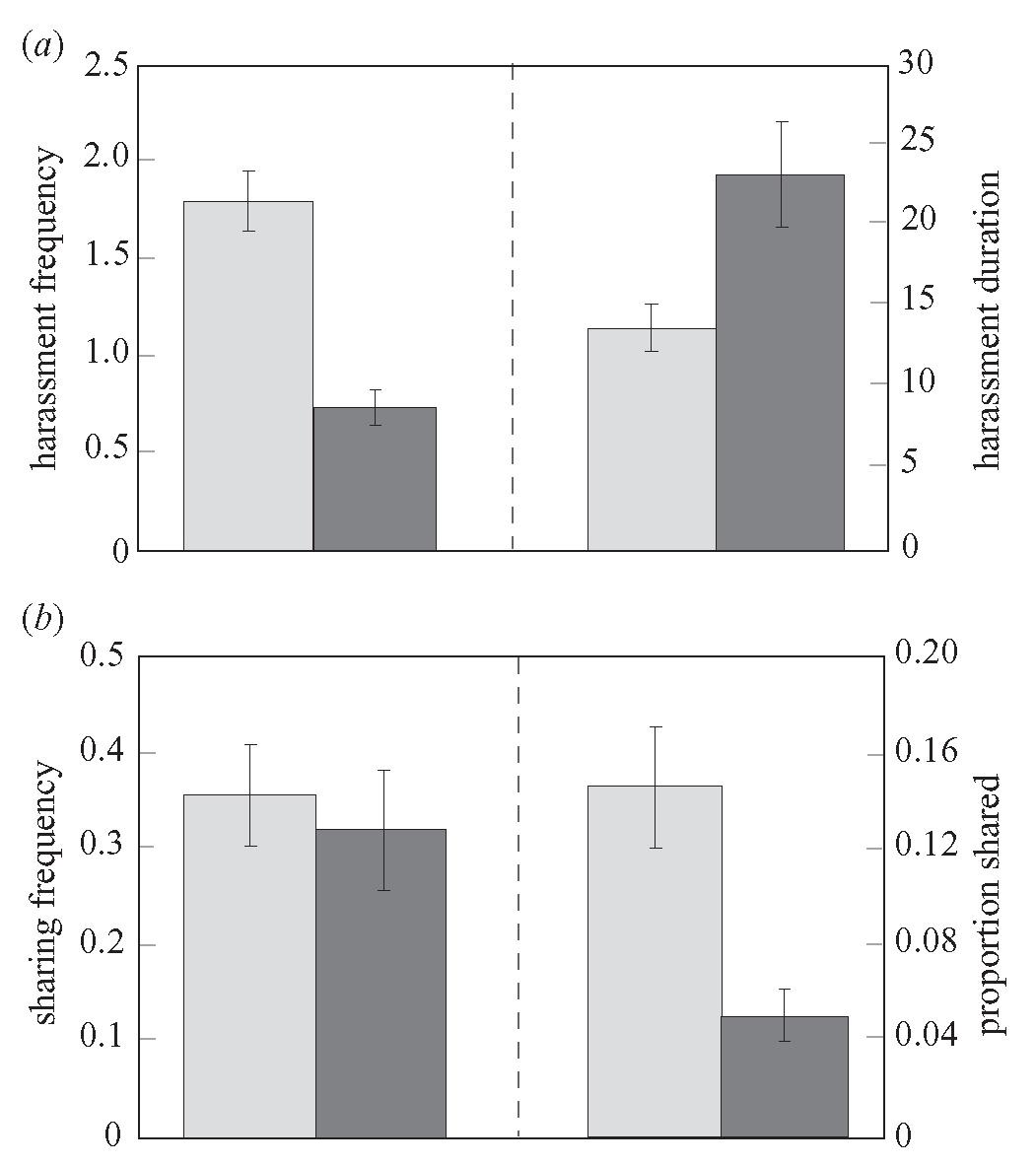
Despite the similarity between these two experiments, they were not identical. Cage size and food amount did not scale exactly to body size. Given these and other subtle differences, we must interpret these comparative results with caution. Nevertheless, repeated-measures ANOVAs reveal striking similarities and differences between chimpanzees and squirrel monkeys. First, the species differed in harassment frequency (F1,6 = 20.69, p = 0.004), with squirrel monkeys harassing three times more frequently than chimpanzees (figure 2a). Chimpanzees, however, showed a non-significant tendency to harass for longer durations (F1,6 = 4.05, p = 0.09).
The most noteworthy result involves sharing. Chimpanzees and squirrel monkeys showed no difference in sharing frequency (F1,6 = 0.04, p = 0.85). Surprisingly, squirrel monkeys shared a slightly larger proportion of their food than chimpanzees (F1,6 = 6.35, p = 0.05). Thus, in this experiment, these two species shared food equally frequently, but squirrel monkeys shared more food with beggars (figure 2b).
Discussion
This study supports the harassment model predictions of Stevens & Stephens (2002) and provides a simple set of experimental procedures for testing other species. The non-contingent benefits of gathering scraps from divisible food increased harassment in chimpanzees, and harassment increased the beggar’s food intake in squirrel monkeys. For both species, the absence of the partition increased harassment by beggars, which increased sharing by owners. A further analysis of No Partition trials suggests that sharing increased in the presence of direct harassment by the beggars—owners shared more frequently as begging intensity increased. These results generate two conclusions: (i) beggar harassment substantially increases food sharing; and (ii) there are few differences in sharing rates between squirrel monkeys and chimpanzees in this experiment (figure 2b).
Although several studies have suggested a relationship between harassment and food sharing (Teleki 1973; Wrangham 1975; Goodall 1986), to the best of the author’s knowledge, this study offers the first empirical test that manipulates the potential for harassment and determines effects on sharing. Therefore, the immediate, selfish benefits of reducing the costs of harassment directly influenced sharing. By imposing costs, beggars manipulate the owners’ fitness benefits of defending a resource. To avoid these costs, food owners must relinquish part of the food that they possess.
(a) Implications of harassment effects
Chimpanzees frequently share food in both natural and captive settings (Feistner & McGrew 1989). In some populations of chimpanzees, food owners almost always share part of a carcass with others following hunts (I. C. Gilby, unpublished data). Chimpanzees also have food calls that are given when individuals find both divisible and non-divisible foods (Hauser et al. 1993). By contrast, squirrel monkeys rarely share food in the wild or in captivity (Fragaszy & Mason 1983), and have no food calls. Despite these apparent differences in sharing rates and vocal behaviour, chimpanzees and squirrel monkeys share equally often, and squirrel monkeys actually share larger proportions of food in this experimental situation (figure 2b). Because harassment elicited sharing in both chimpanzees and squirrel monkeys, it could provide a general explanation of sharing.
Harassment is a parsimonious and broadly applicable explanation of sharing for several reasons. First, sharing provides immediate, selfish benefits for the owner by avoiding costly harassment. Owners do not act altruistically in the short term and wait for future repayment as they do in reciprocally altruistic interactions. These findings support a growing body of mutualistic explanations of cooperation (Clements & Stephens 1995; Grinnell et al. 1995; Clutton-Brock et al. 1999). Second, harassment does not require complex cognitive skills. Unlike reciprocity, individuals need not track discounted future rewards, debts owed, favours given or the likelihood of reciprocation (Stevens & Hauser 2004). Instead, natural selection simply favours individuals that donate food in such a way as to avoid net costs. Finally, harassment does not require special relationships between owner and beggar. In instances of kin-selected food sharing, sharing occurs only within a limited set of related individuals. In reciprocal altruism, individuals must recognize and interact with the same individuals to track reciprocation. In the harassment paradigm, however, individuals need not recognize each other, much less interact repeatedly. Indeed, individuals that have never met and will never meet again may share food.
Harassment may explain many instances of sharing for a number of taxa. To date, researchers have reported harassment and sharing in only a few species, most notably chimpanzees (Stevens & Gilby 2004). Its role in chimpanzee food sharing, however, has probably been vastly underestimated. For example, de Waal (1989) and Mitani & Watts (2001) both discounted the effects of observed harassment and concluded that reciprocity and trade maintained sharing. In addition, Stanford et al. (1994) proposed correlational evidence that males may trade food for sex by sharing more frequently with sexually receptive females. Previously, however, Teleki (1973) provided an alternative explanation of Stanford’s results by demonstrating that receptive females harass food owners more often. Therefore, patterns of reciprocal sharing or trade may result from reciprocal harassment rather than reciprocal altruism (Stevens & Cushman 2004). If individual A frequently begs from individual B and vice versa, they will show a reciprocal pattern, but this pattern can be explained by immediate, selfish benefits rather than by reciprocally altruistic benefits. By underestimating harassment, studies of sharing may ignore a potentially basic mechanism of sharing and misinterpret reciprocal sharing patterns. This, of course, does not imply that harassment is always the best explanation of sharing or that it excludes other explanations—it could act in concert with reciprocity. Rather, harassment must be either ruled out, or statistically controlled for, before invoking more complex explanations such as reciprocity, trade and meat-for-sex.
(b) Natural harassment
These laboratory results on captive species reveal that harassment can influence sharing in primates; however, they do not demonstrate the importance of harassment in natural sharing situations. For chimpanzees, many studies of wild populations have verified the frequency of harassment (Teleki 1973; Wrangham 1975; Goodall 1986). Critically, a recent study focusing on harassment and sharing in wild chimpanzees corroborated the experimental results described here (I. C. Gilby, unpublished data). Gilby found that harassment predicted sharing patterns better than grooming frequency, association levels and female sexual receptivity in chimpanzees at Gombe National Park. No evidence of sharing exists for wild populations of squirrel monkeys. Evidence from another primate species, however, validates the importance of harassment in natural sharing situations. Hauser (1992) described scenarios in which rhesus macaques (Macaca mulatta) discovered food sources and could either withhold or give recruitment calls. If other monkeys discovered an individual that did not call, they chased and attacked the non-caller. If the individual called, others shared the food in relative peace. Interestingly, female callers consumed more food on average than female non-callers, suggesting that calling (and thereby sharing) offered higher benefits than not calling and facing harassment.
The role of harassment in human food sharing remains unclear. Blurton Jones developed the original tolerated theft model to describe patterns of food sharing in humans. Evidence for tolerated theft in human societies comes from work on turtle hunting by the Meriam and large-animal hunting by the Hadza. Bliege-Bird & Bird (1997) found that defending turtle meat is costly to Meriam hunters and that the marginal fitness value decreases with resource size. Similarly, Hawkes (1993, 2000) suggests that Hadza hunters share large kills because not sharing is too costly. Despite these examples, many anthropologists argue that tolerated theft is relatively uncommon in human food-sharing contexts (Kaplan & Hill 1985; Gurven 2004). Many investigations of tolerated theft, however, focus on overly strict assumptions and predictions that are less restrictive in the recent Stevens & Stephens (2002) model of harassment.
(c) Harassment and punishment
Harassment and punishment are closely related models of cooperation, differing in the timing of benefits to the harasser or punisher. Although models of punishment suggest that it may be evolutionarily stable (Boyd & Richerson 1992; Clutton-Brock & Parker 1995; Gintis 2000), empirical evidence is quite rare in non-human animals. Many of the previously touted examples probably fall under harassment rather than punishment because of immediate benefits associated with the sanctions. Perhaps cognitive constraints make punishment difficult for nonhuman animals to implement. For punishment to be profitable the return benefit must be large, or the delay until the next interaction must be small, to balance the effect of temporal discounting of future cooperative benefits (Stephens et al. 2002). In addition, learning rate is critical to the efficacy of punishment. To create an association between punishment and the uncooperative behaviour, the temporal delay between the uncooperative behaviour and the punishment must be short and the number of learning events (future cooperative interactions) must be relatively large. By contrast, harassment circumvents these cognitive constraints by applying the aversive stimulus during the uncooperative behaviour; therefore, cooperation provides immediate benefits. However, harassment may influence future as well as current cooperation, suggesting that it may be a precursor to punishment in some situations. This could have important implications for the evolution of punishment in many types of cooperative situations.
The author thanks Kevin Haley, Linda Brent and the staff of the Southwest Foundation for Biomedical Research for assistance in working with chimpanzees. He also thanks Larry Williams, Christian Abee and the staff of the Squirrel Monkey Breeding Research and Resource for assistance with the squirrel monkeys. Ian Gilby, Kevin Haley, Marc Hauser, David Stephens and two anonymous reviewers offered helpful comments on the manuscript. This project was funded with support from the L. S. B. Leakey Foundation, Alexander P. and Lydia Anderson Fellowship, Animal Behavior Society and Dayton and Wilkie Natural History Funds. This project was approved by the Institutional Animal Care and Use Committee at the University of Minnesota (Animal Subjects Code 0010A67581).
References
Andreoni, J. A., Harbaugh, W. & Vesterlund, L. 2003 The carrot or the stick: rewards, punishments and cooperation. Am. Econ. Rev. 93, 893–902.
Bliege Bird, R. L. & Bird, D. W. 1997 Delayed reciprocity and tolerated theft: the behavioral ecology of food-sharing strategies. Curr. Anthropol. 38, 49–78.
Blurton Jones, N. G. 1984 A selfish origin for human food sharing: tolerated theft. Ethol. Sociobiol. 5, 1–3.
Boyd, R. & Richerson, P. J. 1992 Punishment allows the evolution of cooperation (or anything else) in sizable groups. Ethol. Sociobiol. 13, 171–195.
Boyd, R., Gintis, H., Bowles, S. & Richerson, P. J. 2003 The evolution of altruistic punishment. Proc. Natl Acad. Sci. USA 100, 3531–3535.
Brosnan, S. F. & de Waal, F. B. M. 2002 A proximate perspective on reciprocal altruism. Hum. Nature 13, 129–152.
Clements, K. C. & Stephens, D. W. 1995 Testing models of non-kin cooperation: mutualism and the Prisoner’s Dilemma. Anim. Behav. 50, 527–535.
Clutton-Brock, T. H. & Parker, G. A. 1995 Punishment in animal societies. Nature 373, 209–216.
Clutton-Brock, T. H., O’Riain, M. J., Brotherton, P. N. M., Gaynor, D., Kansky, R., Griffin, A. S. & Manser, M. B. 1999 Selfish sentinels in cooperative mammals. Science 284, 1640–1644.
de Waal, F. B. M. 1989 Food sharing and reciprocal obligations among chimpanzees. J. Hum. Evol. 18, 433–459.
de Waal, F. B. M. 1997 Food transfers through mesh in brown capuchins. J. Comp. Psychol. 111, 370–378.
Fehr, E. & Ga¨chter, S. 2000 Cooperation and punishment in public goods experiments. Am. Econ. Rev. 90, 980–994.
Feistner, A. T. C. & McGrew, W. C. 1989 Food-sharing in primates: a critical review. In Perspectives in primate biology, vol. 3 (ed. P. K. Seth & S. Seth), pp. 21–36. New Delhi: Today & Tomorrow’s Printers and Publishers.
Fragaszy, D. M. & Mason, W. A. 1983 Comparisons of feeding behavior in captive squirrel and titi monkeys (Saimiri sciureus and Callicebus moloch). J. Comp. Psychol. 97, 310–326. Gintis, H. 2000 Strong reciprocity and human sociality. J. Theor. Biol. 206, 169–179.
Goodall, J. 1986 The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Harvard University Press.
Grinnell, J., Packer, C. & Pusey, A. E. 1995 Cooperation in male lions: kinship, reciprocity or mutualism? Anim. Behav. 49, 95–105.
Gurven, M. 2004 To give and to give not: the behavioral ecology of human food transfers. Behav. Brain Sci. (In the press.) Hamilton, W. D. 1964 The genetical evolution of social behaviour. I, II. J. Theor. Biol. 7, 1–52.
Hauser, M. D. 1992 Costs of deception: cheaters are punished in rhesus monkeys (Macaca mulatta). Proc. Natl Acad. Sci. USA 89, 12 137–12 139.
Hauser, M. D., Teixidor, P., Fields, L. & Flaherty, R. 1993 Food-elicited calls in chimpanzees: effects of food quantity and divisibility. Anim. Behav. 45, 817–819.
Hauser, M. D., Chen, M. K., Chen, F. & Chuang, E. 2003
Give unto others: genetically unrelated cotton-top tamarin monkeys preferentially give food to those who altruistically give food back. Proc. R. Soc. Lond. B 270, 2363–2370. (DOI 10.1098/rspb.2003.2509.)
Hawkes, K. 1993 Why hunter-gatherers work: an ancient version of the problem of public goods. Curr. Anthropol. 34, 341–361.
Hawkes, K. 2000 Hunting and the evolution of egalitarian societies: lessons from the Hadza. In Hierarchies in action: cui bono? (ed. M. W. Diehl), pp. 59–83. Carbondale, IL: Center for Archaeological Investigations.
Henrich, J. & Boyd, R. 2001 Why people punish defectors: weak conformist transmission can stabilize costly enforcement of norms in cooperative dilemmas. J. Theor. Biol. 208, 79–89.
Hirshleifer, D. & Rasmusen, E. 1989 Cooperation in a repeated Prisoner’s Dilemma with ostracism. J. Econ. Behav. Organ. 12, 87–106.
Kaplan, H. & Hill, K. 1985 Food sharing among Ache foragers: tests of explanatory hypotheses. Curr. Anthropol. 26, 223–246.
Mesterton-Gibbons, M. & Dugatkin, L. A. 1997 Cooperation and the Prisoner’s Dilemma: towards testable models of mutualism versus reciprocity. Anim. Behav. 54, 551–557.
Mitani, J. C. & Watts, D. P. 2001 Why do chimpanzees hunt and share meat? Anim. Behav. 61, 915–924. Sahlins, M. 1972 Stone age economics. Chicago, IL: Aldine Atherton.
Stanford, C. B., Wallis, J., Matama, H. & Goodall, J. 1994 Patterns of predation by chimpanzees on red colobus monkeys in Gombe National Park, 1982–1991. Am. J. Phys. Anthropol. 94, 213–228.
Stephens, D. W., McLinn, C. M. & Stevens, J. R. 2002 Discounting and reciprocity in an Iterated Prisoner’s Dilemma. Science 298, 2216–2218.
Stevens, J. R. & Cushman, F. A. 2004 Cognitive constraints on reciprocity and tolerated scrounging. Behav. Brain Sci. (In the press.)
Stevens, J. R. & Gilby, I. C. 2004 A conceptual framework for non-kin food sharing: timing and currency of benefits. Anim. Behav. (In the press.)
Stevens, J. R. & Hauser, M. D. 2004 Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn. Sci. (In the press.)
Stevens, J. R. & Stephens, D. W. 2002 Food sharing: a model of manipulation by harassment. Behav. Ecol. 13, 393–400.
Teleki, G. 1973 The predatory behavior of wild chimpanzees. Lewisburg, PA: Bucknell University Press.
Trivers, R. L. 1971 The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57.
Wrangham, R. W. 1975 The behavioural ecology of chimpanzees in Gombe National Park, Tanzania. PhD thesis, Cambridge University, UK.
Zar, J. H. 1999 Biostatistical analysis. Upper Saddle River, NJ: Princeton Hall.